INTRODUCTION
MATERIALS AND METHODS
Chemicals and reagents
Determination of total polyphenol and flavonoids content
Determination of total tannin content
Determination of rutin and quercetin content by HPLC
Statistical analysis
RESULTS AND DISCUSSION
Identification and growth of endophytic bacterium
Phenolic compound and flavonoid content
Total tannin content
Rutin and quercetin content
CONCLUSION
INTRODUCTION
Buckwheat (Fagopyrum spp.) is an annual plant belongs to Polygonacae family. Fagopyrum spp. consists of at least 18 species distribution in the world wide (Ohnishi, 1995). However, only Common buckwheat (Fagopyrum esculentum Moench) and Tartary buckwheat (Fagopyrum tatarucum L. Gaertn.) are of economically important and contain higher nutritional value. This plant contain proteins, carbohydrates, lipids, fibers, vitamins, amino acids and minerals as their basic components. Moreover, buckwheat grains has no gluten, so they are safe for people with celiac disease (Halbrecq et al., 2005; Skerritt, 1986). Tartary buckwheat has higher rutin content than common buckwheat (Kitabayashi et al., 1995a, b). Many people have interest in utilizing of Tartary buckwheat grains and sprouts because of their nutritional and pharmaceutical values. Nowadays, fresh and dried Tartary buckwheat sprouts are attracting a lot of attention from pharmaceutical and food companies, and are applied in the prevention of obesity and diabetes (Lee et al., 2006; Lin et al., 2008). Tartary buckwheat sprouts contents phenolic compounds significantly higher during sprouting stage than matured stage (Sharma et al., 2012; Kim et al., 2008). Rutin including quercetin and catechin is the main polyphenol present in buckwheat (Park et al., 2005).
Tannins is a polyphenolic compound which is proven as antioxidant and possesses a chemo-protective potential found in plant food (Saxena et al., 2013). Gadzo et al. (2010) reported that light exposed leaves had higher concentration of tannins in Tartary buckwheat. On the other hand, the tannin contents significantly increased in the sprouting Tartary buckwheat compared to grains (Lee et al., 2004).
Endophytes are microorganisms that colonize living internal tissues of plants without causing any harm to their host. All types of microorganisms (fungi, bacteria and actinomycetes) have been discovered as endophytes. Studies have revealed the ubiquity of these microbes, with an estimate of at least one million species of endophytic microorganisms residing in plants with protect their hosts from infectious agents and adverse conditions by secreting bioactive secondary metabolites (Hyde & Soytong, 2008; Strobel, 2003). Moreover, there is enormous potential for exploiting these endophytes for medicinal, agricultural and industrial uses (Aly et al., 2010). Therefore, the aim of this study was to investigate the secondary metabolites content of Tartary buckwheat sprouts inoculated with endophytic bacterium Herbaspirillum rubrisubalbicanse isolate at different temperature.
MATERIALS AND METHODS
Endophytic bacterium Herbaspirillum rubrisubalbicans was isolated from Tartary buckwheat stem at the Laboratory of Applied Microbiology, Department of Biology, Faculty of Science, Chiang Mai University, Thailand. The taxonomic studies of the bacteria isolate was determined as described in Bergy’s manual of systematic bacteriology. Gram staining was performed after 24 h of bacteria cultivation at 37°C on nutrient agar media. Endospore formation was assessed with spore-staining method using malachite green (Schaeffer & Fulton, 1993). Colony formation was assessed by spread plate technique on nutrient agar media. The biochemical test including oxidase test, catalase test, methyl red test, Voges-Proskauer test and citrate test were performed according with the method described by MacFaddin (2000). The growth of endophytic bacterium Herbaspirillum rubrisubalbicans was evaluated by using nutrient broth (NB) and nutrient agar (NA) media (Atlas, 2010). Endophytic bacterium cultivation was conducted at 37°C on 150 rpm rotary shaker. Sample was taken every 1h and measured at 600 nm by spectrophotometer. Viable cell was determined by the spread plate method on nutrient agar (NA) media and all plates were incubated at 37°C for 24h. Colony formation was counted and the value was expressed as cfu/ml of sample.
A single colony was selected and inoculated into NB medium, then incubated at 37°C for 8-10 h under shaking conditions. Exponentially growing cells were harvested by centrifugation at 6000 rpm for 20 min, and re-suspended in sterilized normal saline (0.85% NaCl) solutions to obtain the final cell densities of 108 cfu/ml. The inoculums were prepared with the proper diluents by using sterile distilled water to obtain a final concentration of 10, 20 and 40% (v/v), respectively. Tartary buckwheat seeds were surface- sterilized with 2.5% (v/v) sodium hypochlorite for 3 min, and rinsed thoroughly in sterile distilled water for 4 times with 1 min duration. Seeds were soaked in liquid suspension of endophytic bacterium (108 cfu/ml) by separating each treatment for 0, 4, 8 and 12 h, at 30°C (Fig. 1).
Seven days-old Tartary buckwheat sprouts obtained from endophytic bacterium-treated seeds were harvested, cleaned and dried in hot air oven at temperature of 50°C for 24h. The dried samples were ground in a mortar and pestle into powder. The powdered samples (2.0 g) sprouts were taken and 100 ml of 80% methanol was added to each and incubated overnight in a shaker followed by filtration using filter paper No.2. The extract was dried using a rotatory evaporator (Eyela N-100, Japan) at a temperature of 40°C. The extracts were vacuum freeze dried to remove the remaining moisture. The extracts were stored in a refrigerator at -20°C for further analysis.
Chemicals and reagents
The following reagents were used: Folin-Ciocalteu’s phenol reagent (Fluka), Folin-Denis reagent (Fluka), Gallic acid (Sigma-Aldrich), Tannic acid (Fluka), Sodium carbonate (Fisher), Aluminum nitrate nanohydrate (ACS reagent, Sigma- Aldrich), Potassium acetate (Fisher), Methanol (AR, ACI Labscan), Ethanol 95% (AR, ACI Labscan), Deionized water (Thermoscientific), Rutin (Sigma-Aldrich), Quercetin (Sigma- Aldrich), Methanol (HPLC grade), Formic acid (Reagent grade, Sigma-Aldrich). 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) (Sigma-Aldrich), Ferric chloride (Fluka), Ferrozine [Iron (II) chloride (FeCl2)] (Fisher), Potassium ferricyanide (III) (RANKEM), Trichloroacetic acid (ACS reagent, Sigma- Aldrich), Ferric chloride (Fluka), disodium hydrogen phosphate Anhydrous (RANKEM), Sodium dihydrogen phosphate Anhydrous (UNIVAR).
Determination of total polyphenol and flavonoids content
Total polyphenol (TP) content was determined by the Folin-Ciocalteu assay method as described by Eom et al. (2008) with slight modifications. Briefly, an aliquot of 200 μl was added to a test tube containing 200 μl of phenol reagent (1 M). The volume was increased by adding 1.8 ml of distilled deionized water (DDW). Further 400 μl of Na2CO3 (10%, w/v) was added and adjusted final volume to 4 ml by adding DDW. The absorbance was measured at 725 nm. The TP content was expressed as mg of Gallic acid equivalents (GAE) per gram of dried sample.
Total flavonoid (TF) content was determined according to the method described by Eom et al. (2008) with slight modification. Briefly, an aliquot of 0.5 ml was mixed with 0.1 ml of aluminum nitrate (10%, w/v) and 0.1 ml of potassium acetate (1 M). Thereafter, 3.3 ml of 80% methanol was added to make the total volume 4 ml. The mixture was vortexed and incubated for 40 min and the absorbance was measured at 415 nm. Quercetin was used as a standard and the value of TF content was expressed as mg of quercetin equivalent (QE) per gram of dried sample.
Determination of total tannin content
Total tannin content was determined by Folin-Denis method (Saxena et al., 2013). 1 ml of extract was mixed with 7.5 ml of standard solution of tannic acid (10-50 µg/ml). Then 0.5 ml of Folin-Denis reagent and 1 ml of Na2CO3 (10%, w/v) solutions were added. The volume was made up to 10 ml with distilled deionized water. The absorbance was measured at 700 nm in spectrophotometer. Total tannins content was expressed as mg of tannic acid equivalent (TAE) per gram of extract.
Determination of rutin and quercetin content by HPLC
The quantitative estimation of rutin and quercetin were performed by HPLC (Agilent 1100 Series, HPLC Value System, Germany). The analysis was performed according to the procedure reported by Rangkadilok et al. (2005) and Zvezdanovic et al. (2012). The HPLC instrument and operating conditions for rutin and quercetin was as follows: injection volume 10 µl, flow rate 1.0 ml/ min, UV detector 270 nm and Zorbax C18 ABS (150 × 4.6 ㎜. i.d., 5 µm) reversed phased column with two mobile phase (A): Formic acid (0.4%) and (B): Methanol (100%).
Statistical analysis
Statistical analysis of all tests were carried out using Statistix software version 8.0. FL. Data was analyzed with ANOVA and Least significant difference (LSD) test at P ≤ 0.05 level was used to separate the means when the ANOVA F-test indicated a significant effect of the treatments.
RESULTS AND DISCUSSION
Identification and growth of endophytic bacterium
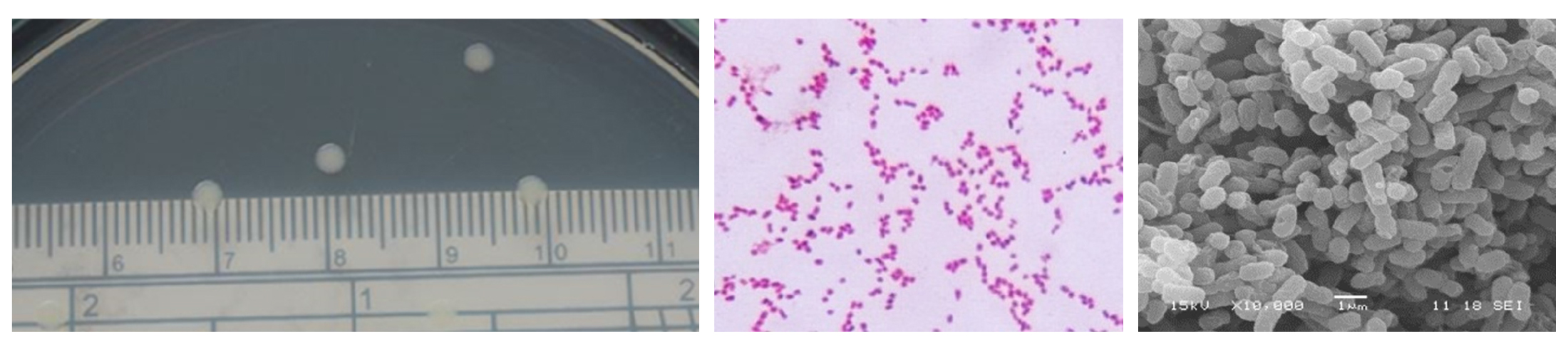
The morphological characteristics and biochemical test of Herbaspirillum rubrisubalbicans were shown in Fig. 1 and Table 1. Cells of H. rubrisubalbicans exhibited gram-negative staining and negative staining endospore. For biochemical tests, negative test in methyl red test, indole test and oxidase test, while the positive test occurred with Vogas-Prokauer test, citrate utilization test and catalase test. However, the morphological characteristics, they generally have a circular form, convex elevation, entire margin and rode shape (Table 1). Colony formation with a small moist white colony and smooth with rode shapes was found in NB medium (Fig. 1). The optimal condition for H. rubrisubalbicans growth was performed using nutrient broth and nutrient agar medium. It is reported that endophytes have beneficial effects on plant growth, improvement of plant resistance to environmental stress and enhance N2 fixation (Spiering et al., 2006; Briatia et al., 2016). The exponential growth phases was 8-10h of incubation with a cell density 108 cfu/ml, moreover, viable cell was more than 300 cfu/ml after 48h of incubation times (data not shown).
Phenolic compound and flavonoid content
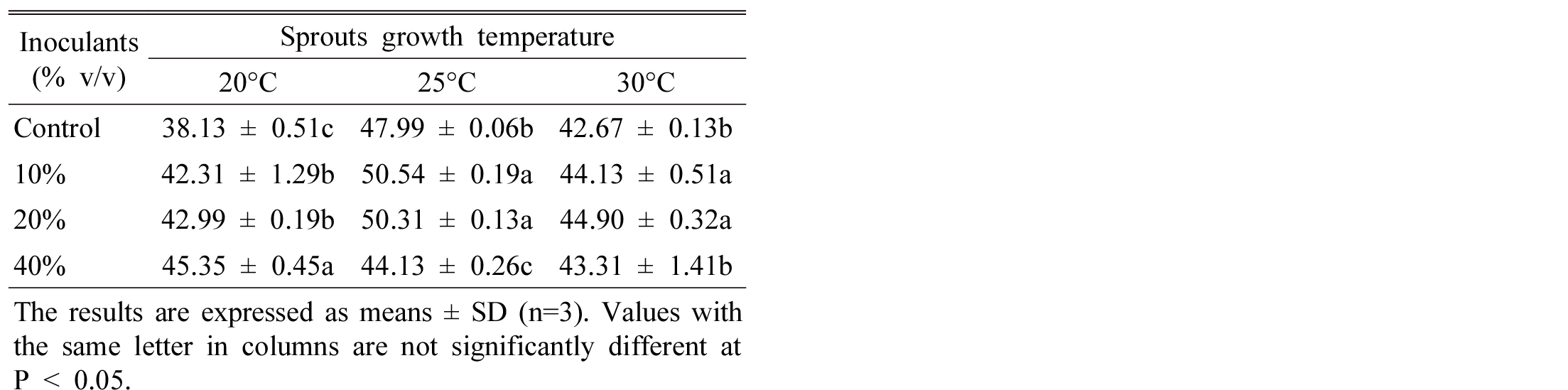
It is shown that seed inoculation significantly increased total polyphenol (TP) and flavonoid content of Tartary buckwheat sprouts compared to control at 20°C growth temperature. However, increasing temperature enhanced TP content at lower inoculant rate (Table 2). Generally, temperature has an enormous positive effect to accumulation of secondary metabolites in plant (Shiow and Zheng, 2001). However, Chen et al. (2010) reported that plant inoculated with S. cinereus had the highest total polyphenols content of tea leaves. Aquaculture buckwheat sprouts significantly enriched total polyphenolics content higher than that of solid-phase cultured to 13% (Peng et al., 2009).
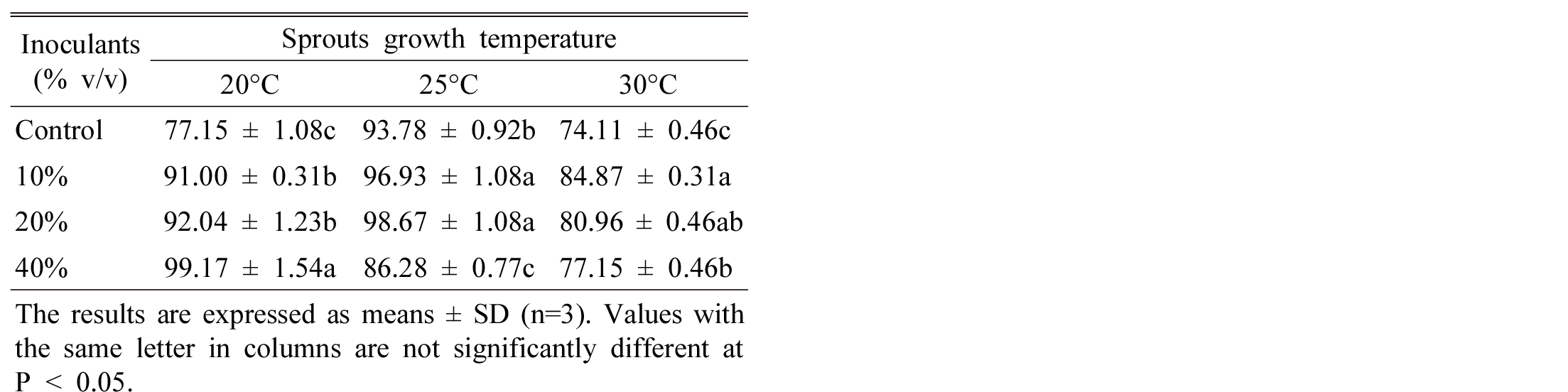
The content of total flavonoid of sprouts was high in order, 40% > 20% > 10% > control at 20°C, however, the order is different where 10% and 20% inoculation has same trends for flavonoid content at 25 and 30°C (Table 3). From these results it is commercially economic to consider lower growth temperature regarding inoculation ratio. Tao et al. (2004) shown that Tartary buckwheat production by application of microbial inoculants (Bacillus megaterium) effectively increased flavonoids content. Furthermore, they suggested that Streptomyces strain could be adopted in the production of Tartary buckwheat sprout to get better quality buckwheat tea. Randhir et al. (2008) found lower phenolic content in buckwheat seedling when grown under high temperature. Li et al. (2010) reported that after treated Tartary buckwheat by endophyte strains N3, the content of flavonoids reached 4.59% and the production of flavonoids was 15.3% higher than the control.
Total tannin content
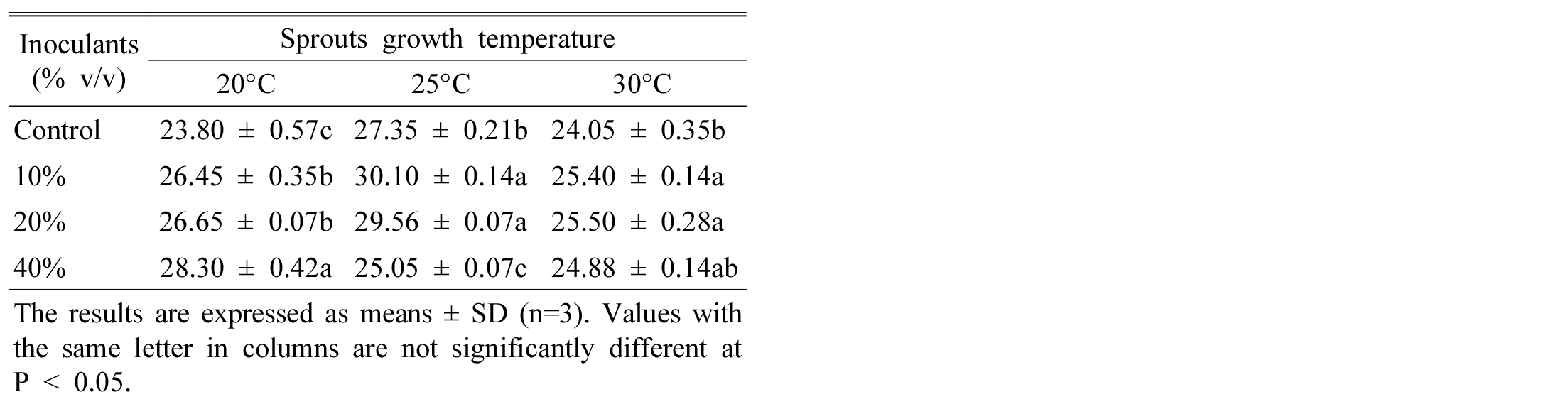
Results of total tannin content is presented in Table 4. At 20°C growth temperature total tannin content significantly increased when the inoculation rate was 40%. However tannin content increased with increasing temperature regardless inoculant rate. Buckwheat sprout is known as a rich source of tannin (Kreft et al., 2002). Previous report explored that tannin content highly affected by Bradyrhizobium inoculation in cowpea seeds (Musa et al., 2011). Elsheikh (2001) also demonstrated that Rhizobium inoculation of legumes seed significantly increased tannin content. However, higher tannin content was achieved in 25°C temperature in our study. Similar results were obtained by Wu et al. (2016), they found higher tannin content of sorghum grain when grown under high temperature. In the same way Trifoli leaf was accumulated higher condensed tannin when grown in high temperature (Lees et al., 1994).
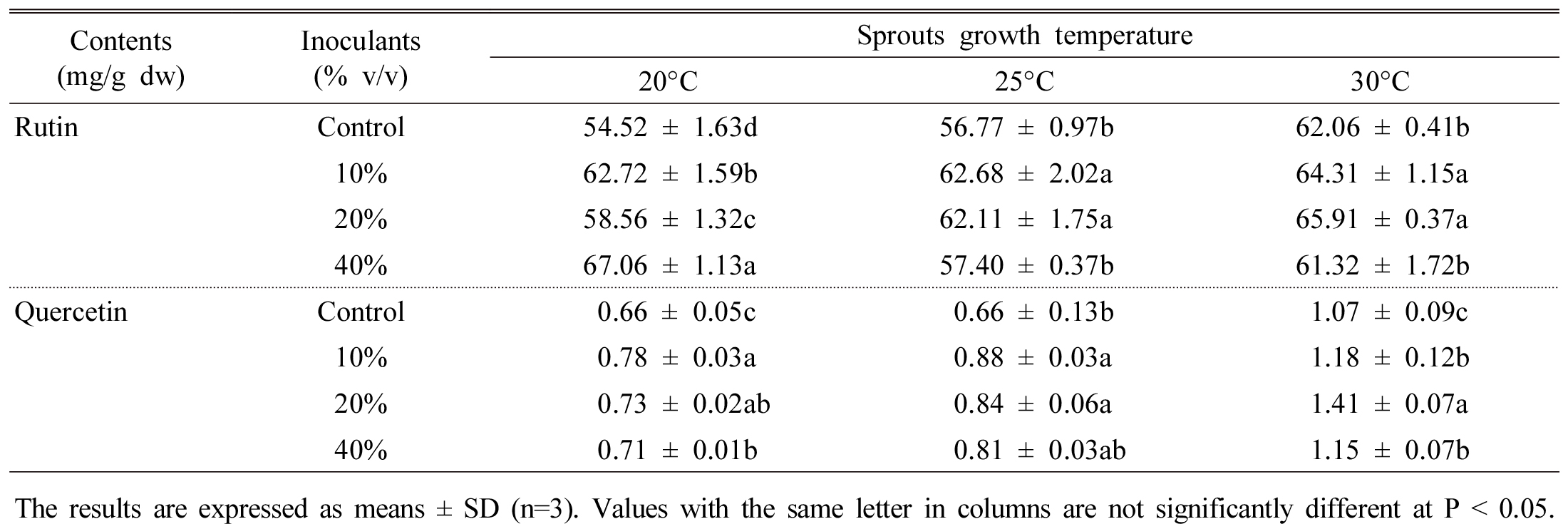
Rutin and quercetin content
It is observed that rutin content was highest at 40% inoculation rate at 20°C temperature, however, quercetin content was increased at 20% inoculation rate at 30°C temperature (Table 5). The chemical structure shows that rutin has sugar moiety in their ring, on the other hand, quercetin form without this moiety. High temperature may destructed the sugar moiety from rutin therefore, rutin converted to quercetin therefore enhanced quercetin was achieved. Report showed that seed inoculation with microbes (Streptomyces jingyangesis) significantly increased rutin content in tartary buckwheat (Tao et al., 2004). Similar results were obtained by Singh et al. (2016) in rutin content from rice. They observed that seed inoculation with Bacillus subtilis greatly increased rutin and other phenolic content from rice seed.
The enriched phenolic compounds can be the reason for the enhanced biofilm formation which has been implicated to play a key role in efficient root colonization. Exerting chemical from microbes in plant root zone might have beneficial effects on plant secondary metabolites. Study showed that effective microbial colonization in Arabidopsis thaliana roots was triggered by certain plant polysaccharides (Beauregard et al., 2013). Similarly, in another study L-malic acid (MA) secreted from roots of A. thaliana and recruited the beneficial rhizobacterium B. subtilis thus accelerate to accumulates plant secondary metabolites.
CONCLUSION
It is observed from our findings that inoculation rate is depended upon plant growth environment. Higher inoculant rate is effective when sprouts are grown at lower temperature. Inoculated plant grown under elevated temperature is giving same output as low temperature dose. As therefore, it might be suggested that Tartary buckwheat inoculated with Herbaspirillum spp should be grown under lower temperature in order to get higher secondary metabolites.