INTRODUCTION
MATERIALS AND METHODS
Rice cultivars and high temperature treatments
Flowering pattern
Pollen viability rate
Pollen germination rate
Fertility rate
Statistical analysis
RESULTS
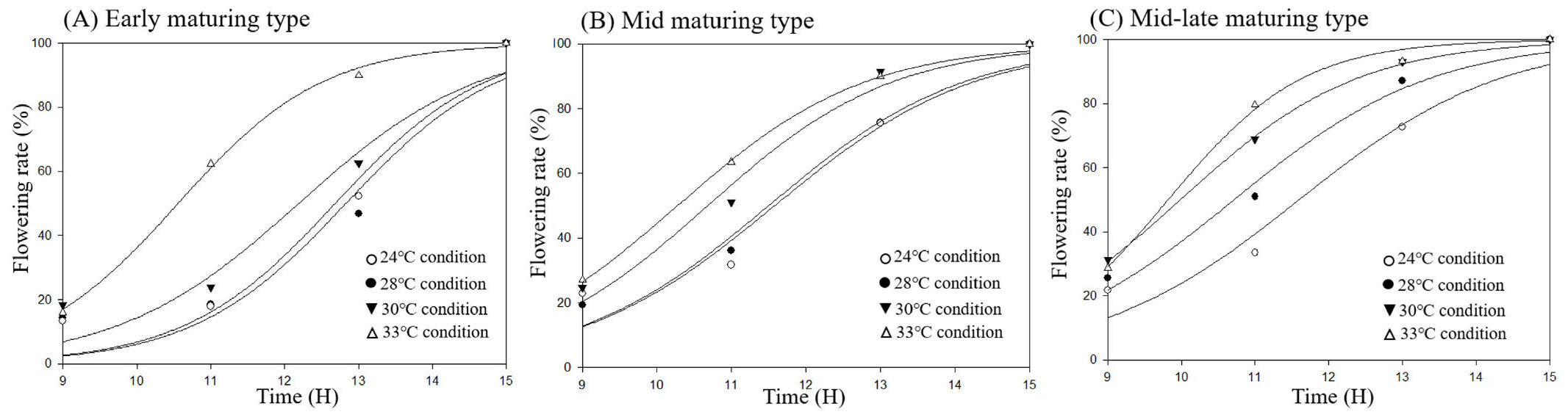
Influence of high temperature on flowering pattern in Korean rice cultivars
Effect of high temperature to pollen viability and germination
Effect of high temperature to fertility rate
DISCUSSIONS
CONCLUSION
INTRODUCTION
In the future, the new challenge for crop production will include climate change and its consequences (FAO, 2019). Extreme temperature will likely occur with more frequency and intensity under the impact of climate change, consequently presenting more challenge for future crop development (IPCC, 2014; Siddik et al., 2019). The global air temperature is predicted to rise by 0.2~0.4°C per decade, which leads to temperature 1.8~4.3°C higher than the current temperature in 22nd century. The warming trend and increasing temperature extremes have been observed across most Asia region over past century (IPCC, 2014). In South Korea, the maximum air temperature over 35°C and peaked to 39.4°C in summer have been frequently recorded (Met Office, 2011).
The major crops such as rice, maize and wheat have been reported yield loss by high temperature (Lobell et al., 2011). Heat stress is defined as the rise in temperature beyond a critical threshold for a period of time that is sufficient to cause irreversible damage to plant growth and development (Fu et al., 2016). By warming trend, Korea’s rice yield is predicted to decrease by 5% at some inland plain areas by 2040 and reached 10% or higher by 2071-2100 (Kim et al., 2010).
The response of plants to temperature stress depends on duration, intensity, period of its occurrence (day or night), rate of temperature’s change and the developmental stage (Shad et al., 2018). A rise in temperature beyond a critical threshold for a period of time sufficient to cause irreversible damage to plant growth and developments which defined as heat stress (Krishnan et al., 2011). Heat stress causes serious adverse effects on rice production (Shi et al., 2017). The ideal temperature ranges for maintaining the rice yield is from 27 to 32°C, however, temperature above 32°C negatively affect all stage of rice plant growth and development (Aghamolki et al., 2014).
Flowering periods (anthesis and fertilization) have been identified as the most sensitive stage to high temperature condition (Tan et al., 1985; Yang et al., 2017). A threshold proposed for heat susceptible cultivars is 35°C while the threshold 38°C is more appropriate to evaluate true heat tolerant variety (Yoshida et al., 1981). According to Wang et al. (2019), the spikelet fertility rate declined by over 15% when rice flowering period encountered with a period of high temperature above 38°C.
In rice, the reproductive process (dehiscence of the anther, shedding of the pollen, germination of pollen grains on stigma) occurs within 1 hour after reproductive, that are more sensitive to high temperature and disrupt at day temperature over 33°C (Satake & Yoshida, 1978). High temperature above 35°C at the flowering stage inhibits anther dehiscence and thus result in lower pollen shed on the stigma leading to incomplete fertilization (Jagadish et al., 2010). The cause for decreasing pollen viability is presumably by an imbalance in protein expression, abandoned biosynthetic, partitioning and translocation of soluble sugars, imbalance in photo-hormones release and loss of pollen water content (Shad et al., 2018).
High temperature induced spikelet sterility has been reported in Thailand (Osada et al., 1973), China (Tian et al., 2010) and Japan (Ishimaru et al., 2010). However, the rare investigation in the effect of heat stress on pollen quality, spikelet fertility at the flowering stage in Korean rice cultivars has been elucidated. In addition, most previous studies of the effect of high temperature on rice are limited to constant elevated temperature in growth chamber. These approaches are limited because the temperature in natural condition changes time to time in a day. In this study, eleven cultivars were subjected to different temperature regimes during flowering period to evaluate the response in pollen characteristics and seed set by sunlit phytotron’s condition which is imitated due to the changes in natural condition.
MATERIALS AND METHODS
Rice cultivars and high temperature treatments
To analyze high temperature response of rice during flowering time, we selected 11 rice cultivars which are being widely cultivated in South Korea in these day. Among 11 cultivars, we selected 3 cultivars in early maturing type, 4 cultivars in mid-maturing type and 4 cultivars in mid-late maturing type (Table 1). Seeds were sowed in the nursery. After 15 days, seedlings were transplanted to 1/5000a Wanger pot (3 hill/pot) with 10 replications. Fertilizer were applied at rate 9N:4.5K:5.7P (kg/10a) as the standard of National Crop Science Institute, Korea. Each pot was provided the fertilizers as a basal at one day before transplanting. These pots were maintained under natural condition.
Table 1. Cultivars used to analyze flowering habits in response to high temperatures.
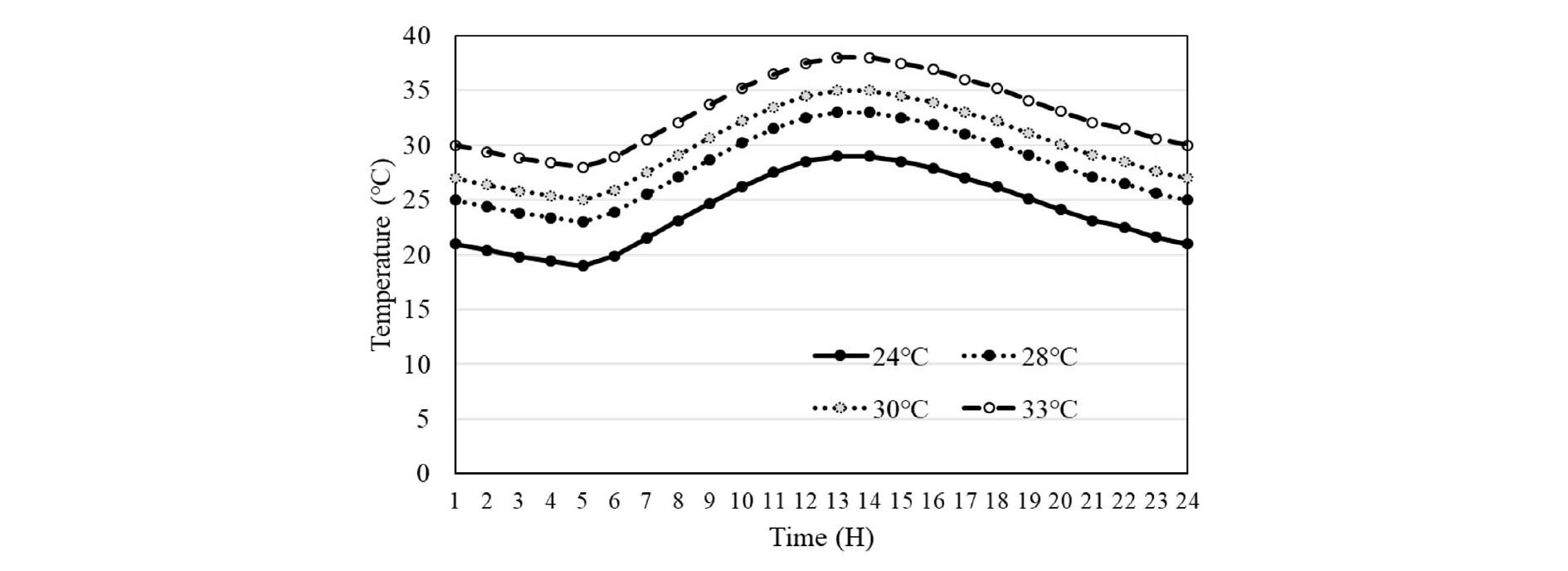
After heading, plants were transferred to sun-lit phytotrons with different average temperature regimes; 24, 28, 30 and 33°C during the flowering stage. Four sun-lit phytotrons were used for the temperature treatment which were imitated to the change in day of natural condition. After the heat treatments, plants will be moved back to the natural condition.
Mean daily air temperature inside phytotrons was maintained very close to the target 24, 28, 30 and 33°C. The hour temperature trend was the same in four regimes which peaked to the highest between 13.00 and 14.00 h (Fig. 1). In the average 24 and 28°C condition, the maximum temperature always was lower 35°C. In the average 30°C condition, the temperature reached 35°C between 13.00 and 14.00 h. In contrarily, the temperature over 35°C was kept a long time in the average 33°C (from 10.00 to 16.00 h). The temperature gap between these conditions was kept constantly in each hour.
Flowering pattern
The plants were transferred from the natural condition immediately before anthesis to phytotron setting temperature at average 24, 28, 30, 33°C. The number of spikelet at anthesis (anther extruding or spikelet gaping and anther visible) were painted with different color every 2 hours between 09.00 and 15.00 h. To record these traits, the spikelets were grouped based on the different color.
Pollen viability rate
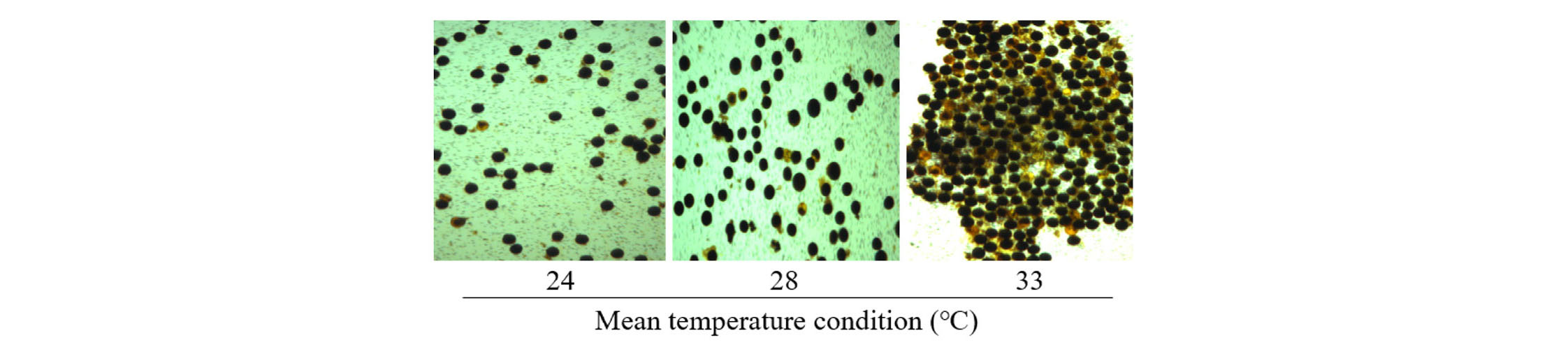
Mature pollen grains were collected from the spikelets before flowering at 4 days after heat stress. Pollen viability was determined by modifying the method of Wang et al. (2006). Pollen grains were collected from anther in the early morning and gather in tubes and stained by 1% iodine potassium iodide (I2-KI). The pollen viability was examined under optical microscope base on staining and morphology. Pollen grains with round shape and stained black color were considered to be viable. Pollen viability was estimated as the ratio of number of stained pollen to number of pollen grains.
Pollen germination rate
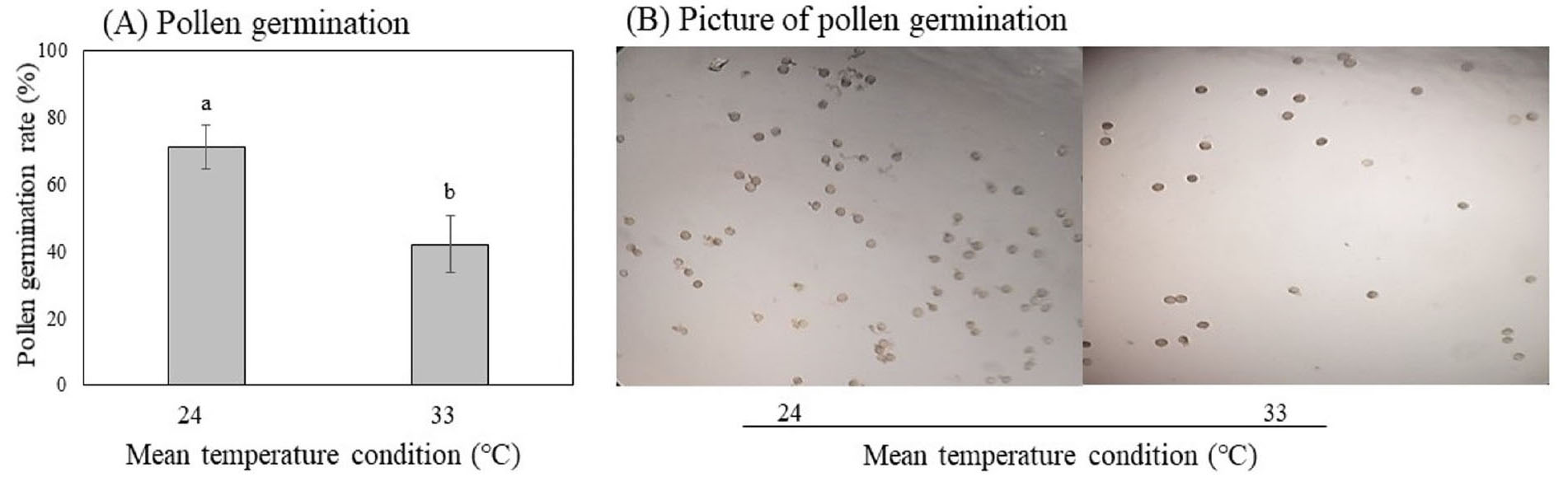
Pollen of each cultivar was collected from freshly opened flowers. Pollen grains from two spikelets at anthesis were dusted over the pollen germination (PG) medium. The Petri dish was opened one by one to prevent pollen from getting into other Petri dishes. The inside humidity was maintained by lining with moist filter paper. There replications of each cultivar were treated at different temperature regimes in 3 h (12.00~15.00 h). The pollen germination was observed in a transmitted light microscope. Pollen was considered germinated if the length of the pollen tube was equal to or greater than the diameter of the pollen grain (Coast et al., 2015). PG was calculated as the percentage of germinated pollen grains to the total number of pollen grains.
Fertility rate
The plants were transferred to high temperature treatment during flowering stage and moved back to natural condition. 15~17 days after heading, the sterility examination was performed by pressing individual to determine the ovary development (filled or not). Both partially and fully filled spikelets were categorized as filled spikelets. Spikelet fertility was calculated as the ratio of filled spikelets to total number of spikelets.
Statistical analysis
SAS version 9.2 (SPSS Inc) was used for data analysis. Duncan’s multiple range test (DMRT) was carried out to identify significant differences (P < 0.05) between individual treatments.
RESULTS
Influence of high temperature on flowering pattern in Korean rice cultivars
In this study, the pattern of flowering in Korean rice cultivars reached anthesis earlier in the morning when the day temperature was warmer (Fig. 2). Three Korean rice groups in maturing type have the same model in flowering responding to different temperature regime. This flowering trend was faster when the average temperature increased. Before 09.00 h, flowering percentage started slightly difference in the order 33>30>28>24°C condition. In average 33°C condition, more than 60% spikelets reach anthesis before 11.00 h and ended before 15.00 h in all Korean rice cultivars.
Effect of high temperature to pollen viability and germination
Pollen grains are in round shape and stained black color that considered to be viable (Fig. 3). The percentage of pollen viability in 4 different temperature conditions of three different groups in maturing type is showed in Table 2. There is no significant difference in pollen viability at average 24, 28 and 30°C in three groups. The percentage of pollen viability in average 33°C reduced significantly compared to lower temperature regime in early maturing type. In mid and mid-late maturing type, the percentage of pollen viability in average 33°C decreased insignificantly than its in other regimes. The pollen viability was 57.9~72.1% in average 33°C condition. In 24, 28 and 30°C, the viable pollen remained more than 70% in all groups of maturing type.
Table 2. Pollen viability percentage at different temperatures in each heading type. Different letters indicate significant differences at p≤0.05.
Pollen was considered germinated if the length of the pollen tube was equal or greater than the diameter of the pollen grain. The pollen germination effect dramatically by high temperature (Fig. 4). There are a significantly decrease in pollen germination at high temperature (33°C) compared to lower temperature (24°C). In average 24 and 33°C condition, the pollen germination rate was 69% and 40% of tested pollen, respectively.
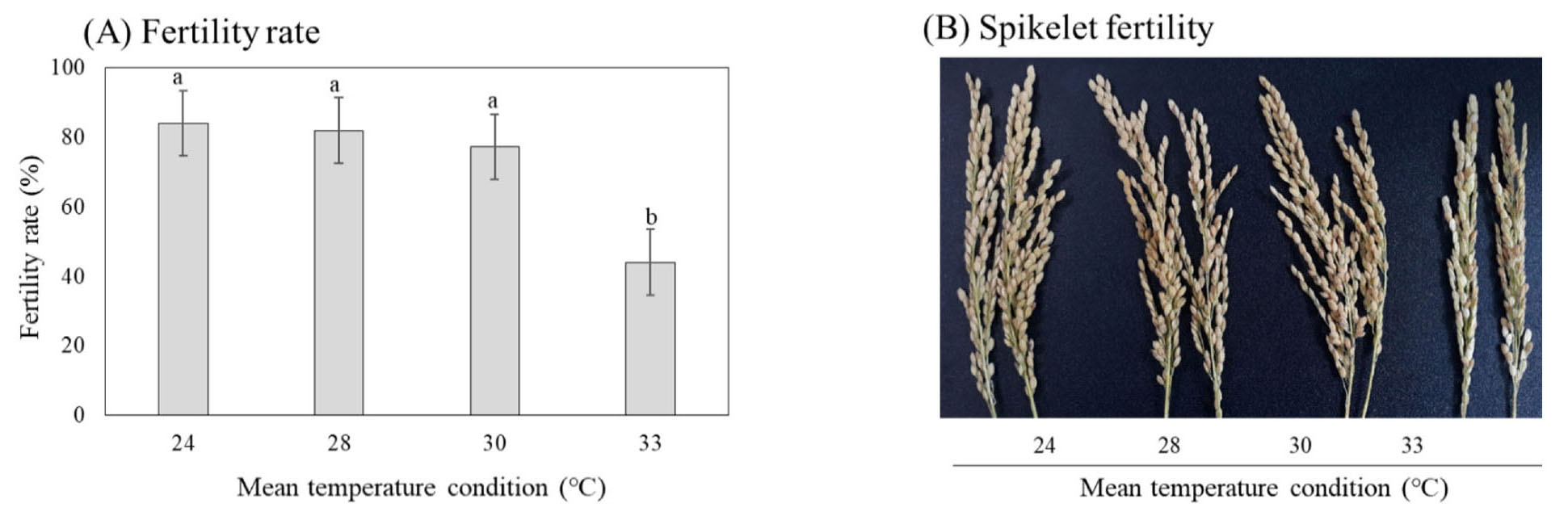
Effect of high temperature to fertility rate
The same responding trend in spikelet fertility to different temperatures was recorded in three groups of maturing type (data not show). Our results indicate the significant differences between the fertility rate between 33°C to lower temperature conditions (Fig. 5). In 24, 28 and 30°C conditions, there was no significant difference in fertility rate which remained more than 80%. The spikelet fertility of Korean rice cultivars in 33°C condition dropped to 40%. The fertility rate in 33°C condition decreased significantly compared to lower temperature conditions.
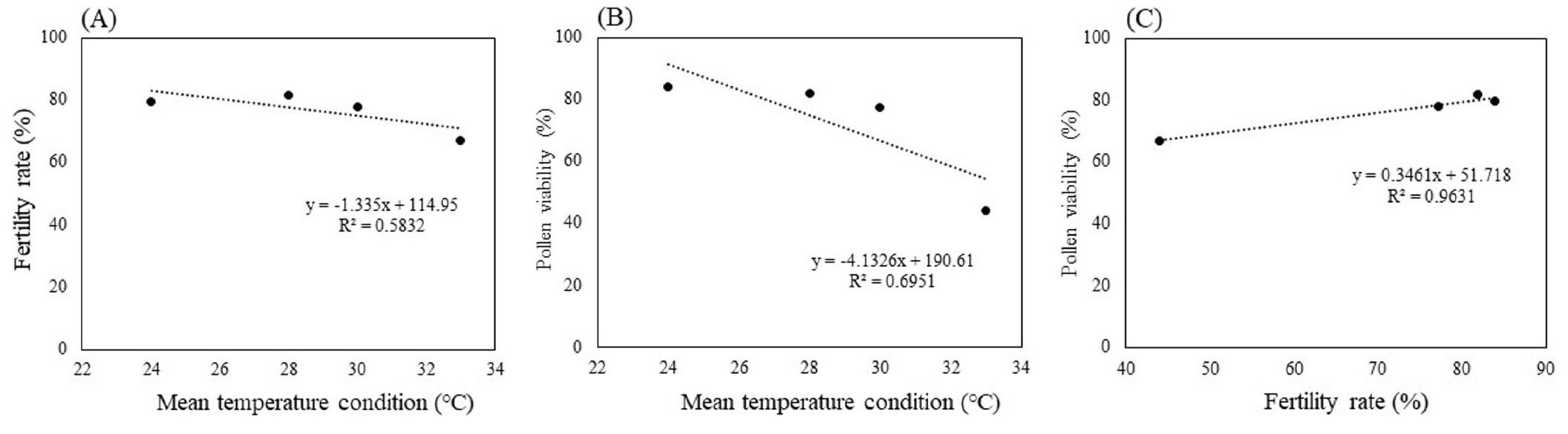
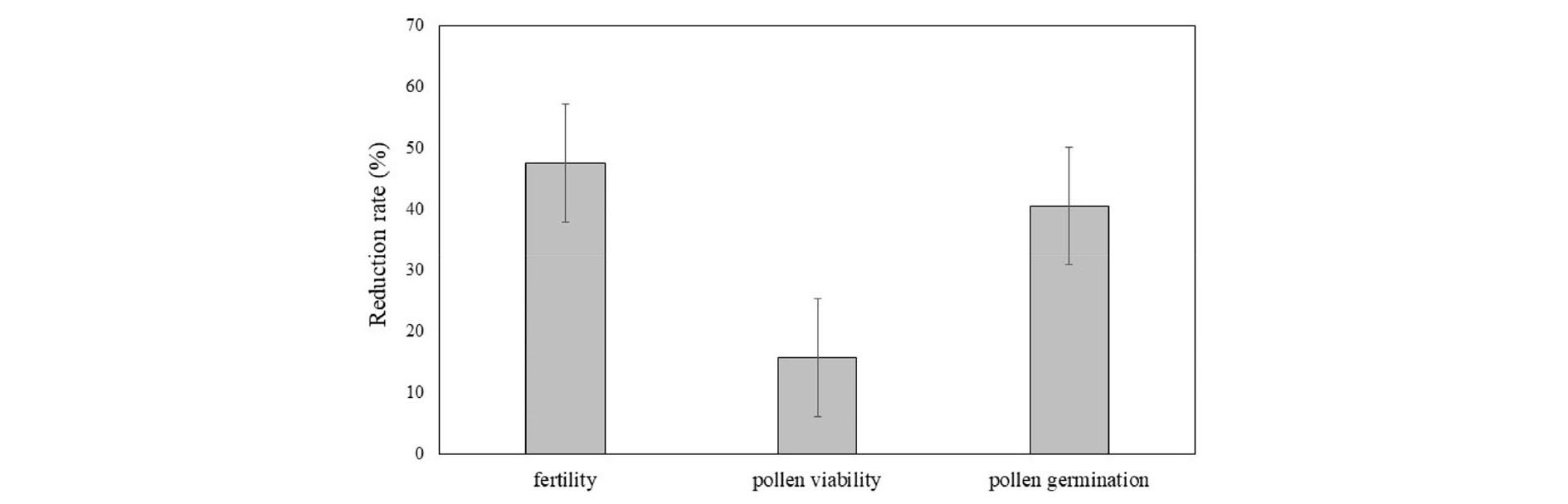
The significant correlation (R2 = 0.96) was recorded between fertility rate and pollen viability, the fertility rate reduced when the percentage of pollen viability decreased (Fig. 6). The pollen viability and fertility rate reduced corresponding to mean temperature increased. The reduction in pollen viability and pollen germination and result in decreasing in fertility was recorded in high temperature condition (33°C) compared to lower temperature (24°C) (Fig. 7). On average, the spikelet fertility rate at high temperature (33°C) shows decrease 47% compared to lower temperature (24°C). The high temperature at flowering time induced nearly 20% reduction of pollen viability. The high temperature after anthesis induced 44% pollen couldn’t germinate.
DISCUSSIONS
Flowering stage in most Oryza sativa genotypes occur over a 5 days period, and the anthesis varies in different rice genotype (Prasad et al., 2006). Due to the large variability in the time of anthesis during different days, further research over entire flowering period is necessary to establish accurate time of day of anthesis. The time at anthesis during the day is important due to the fact that spikelet sterility is induced by high temperature during or soon after anthesis (1~3 h in rice) (Satake & Yoshida, 1978). In this study, more than 60% spikelets of Korean rice cultivars reach anthesis before 11.00 h and ended before 15.00 h at average 33°C, the temperature on this time reach more than 35°C. In rice production, temperature above 35°C at flowering stage induce floret sterility (Yoshida et al., 1981). In this study, most spikelet’s anthesis in average 33°C condition at the disadvantage temperature for spikelet’s fertility. All spikelets at 24, 28, 30°C condition reached anthesis later in the lower temperature compared to 33°C condition.
For single rice spikelet, from opening to pollination happen from 0.5 to 1.0 h, and the flowering period is the most sensitive stage to high temperature (Wang et al., 2019). Sterility in rice is invariably associated with low numbers of pollen or germinated pollen on the stigma (Matsui et al., 2000; Prasad et al., 2006). There is a genotype variation in responding to temperature for cardinal temperature (minimum temperature limit, optimum temperature and maximum temperature limit), pollen germination percentage and maximum pollen length (Kakani et al., 2005). In the report of Coast et al. (2014), the optimum temperature for pollen germination varies by genotype from 25 to 29°C, and the maximum temperature limit 50% germination varies from 31~36°C. The present findings were consistent with the previous findings, whereas the pollen germination reduce more than half when the temperature reached more than 35°C in average 33°C compared to average 24°C condition.
Pollen activity is more influenced by high temperatures before spikelet flowering. The decrease of pollen activity under high temperatures is induced by the stunted development of pollen mother cells and abnormal decomposition of the tapetum (Kumar et al., 2015). High temperature also inhibit anther filling at the panicle initiation stage, which lead to a decline in pollen viability (Wang et al., 2016). In this study, the pollen viability decreases significantly when the anthesis under the temperature above 35°C. In this study, the heat stress caused the damage on the pollen viability and pollen germination that was consistent with the research of Fu et al. (2016).
In the previous studies, the decrease in spikelet fertility was not a result of decreased photosynthesis at high temperature (Prasad et al., 2006). Korean rice cultivars showed the same results with previous studies (Das et al., 2014; Kurma et al., 2015), the spikelet sterility happens through pollen viability and impending pollen tube germination at flowering stage. In the research of Tan et al. (1985), the average temperature above 30°C was determined to be critical for determining rice spikelet sterility. Thus, there is no significant difference in spikelet sterility between average 24, 28 and 30°C. The reduction in spikelet fertility was probably due to high temperature at full flowering (Kumar et al., 2015).
CONCLUSION
The upward annual average temperature trend was recorded at a rate of 0.23°C per decade in South Korea (Jung et al., 2002). In the recent year, the number of day with air temperature over 35°C have been frequently recorded and peaked to 39.4°C more often in summer (Met Office, 2011). Understanding the effect of high temperature to rice fertility is important to maintaining the high yield. In our studies, small different in pollen viability, pollen germination and spikelet fertility rate between average 24, 28 and 30°C condition. But, in the average 33°C condition, the pollen viability and pollen germination reduced dramatically compared to lower temperature. The average 33°C is a critical temperature lead to around 50% spikelet sterility in Korean rice cultivars. The rice spikelet fertility was greatly reduced in the high temperature at flowering stage as a result of decline in pollen viability and pollen germination. Thus the improvement of heat stress tolerance in the process after the pollen germination as well as that in the pollination process is required for high percent fertility under high temperature condition of future circumstance.