INTRODUCTION
MATERIALS AND METHODS
Seed materials and cultivation
Monitoring of soybean growth, development, and yield components
Measurement of isoflavone
Seed visual quality determination
Statistical analysis
RESULTS
Effects of sowing date on flowering (STF) and maturing (FTM) duration
Effects of sowing date on plant height (PH)
Effects of sowing date on the number of nodes, branches, and pods
Effects of sowing date on seed yield and 100-seed weight (HSW)
Isoflavone content variation by sowing date
Seed visual quality/disease infection
Correlation between sowing date and agronomic traits and seed quality
DISCUSSION
CONCLUSIONS
INTRODUCTION
Soybean is a photophilic and thermophilic crop with short-day photoperiodism. Identification of right sowing time is an important factor towards optimizing the impact of weather conditions on soybean yields. Proper adjustment of soybean sowing time can minimize the yield reduction owing to unfavorable weather conditions (Mourtzinis et al., 2019). Among different factors of weather, temperature, photoperiod, and their interactions the most important ones for soybean growing (Câmara et al., 1997; Constable & Rose, 1988). Combined effects of temperature and photoperiod interact with the soybean genotype to regulate growth and development (Cober & Voldeng, 2001; Heatherly & Elmore, 2016) and induce flowering (Rockenbach et al., 2016; Wu et al., 2015). Similarly, the course of thermal and humidity conditions during vegetative stages influence the soybean yields (Below, 2015; Haegele & Below, 2013).
Several studies on the effects of different sowing dates on yield and seed qualities of soybeans have been conducted in different regions, especially, since the early 1900s (Egli & Cornelius, 2009) and it has been regarded as a major management decision influencing soybean growth (Cooper, 2003; Wilcox & Frankenberger, 1987) and yield. Early sowing in October reduced seed quality due to the adverse humid conditions and the threshing mechanical damages, resulting in occurrence of Phomopsis sojae (Pereira et al., 2000). In the same study conducted at Goiânia, Goiás, Brazil, the delayed sowing at the end of December caused a high threshing mechanical damages, deteriorating the seed quality. The variation in planting dates did not affect the visual seed quality and disease infection on seeds (Bajaj et al., 2008). However, maturity group and genotype had substantial effect; late-maturing genotypes exhibited lower of disease-infected seeds and higher visual seed quality compared to their early-maturing genotypes (Bajaj et al., 2008).
The optimal soybean sowing date is a key factor affecting the growth and yield, and it changes with the climate conditions and the associated reactions of cultivars to the day length (Bastidas et al., 2008; Sincik et al., 2011). The information on if cultivar selection and sowing time can enhance the seed quality in early production system is useful in many cases. For instance, in the Mid-south of the US, the early soybean production system using early-maturing cultivars has not been well developed because of lack of stable and well-adapted genotypes with high yield, viability, and vigor (Bajaj et al., 2008).
Sowing date studies have been proved to be advantageous to understand the effect of early planting practice. A yield reduction of 16 kg/ha for each day delay in seed sowing from April 27 to June 22 in Manitoba, Canada and North Dakota, USA was more pertinent to calendar date than to soil temperature (Tkachuk, 2017). The yield reduction due to earlier sowing was well reflected in some parts of the US, such as Wisconsin, where a 21.2 kg/ha/d yield decline was observed when planted from early May to mid-June (Gaspar & Conley, 2015).
Seed sowing on 5 May produced the highest seed yield as well as heaviest 100-seed weight than did sowing on April 20, May 20, and June 5 in Kafr El-Sheikh, Egypt (Kandil et al., 2012). Delaying planting from late April or early May to June or July in southeastern USA increased the seed protein content (Kane et al., 1997). However, the effect of sowing date on seed protein concentration was not consistent in Nebraska (Bastidas et al., 2008).
Many soybean producers may switch their soybean cultivation schedule using different varieties of varying maturity considering the production of preceding and/or succeeding crops. In some cases, selection of early maturing soybeans may result in reduced yield and/or seed qualities. Information on rational adjustment of varietal selection and planting dates can help enhance the yield and seed qualities.
The objective of this study was to investigate the effect of seed sowing date on the growth, development, yield, seed size, isoflavone content, and visual seed quality of soybean with different maturing periods. This study also provides useful information for agronomic traits variation and response of early- and late-growth representative cultivars in Korea.
MATERIALS AND METHODS
Seed materials and cultivation
Three Korean soybean cultivars with varied maturity (Hwangkeumol: early maturing and Daewonkong and Pungsannamulkong: late maturing) were selected. The soybean seeds were obtained from the National Institute of Crop Science, Rural Development Administration (RDA), Miryang, Korea. All cultivars were grown in the fields at the Department of Southern Area Crop Science, Daegu experiment station (35°54’24”N, 128°26’51”E) in 2013 and 2014. The seeds were sown on nine different dates: May 5, 15, and 25, June 5, 15, and 25, and July 5, 15, and 25. Experiment design was factorial experiments for two factors which were 9 levels of seeding dates and 3 levels of cultivars. Cultivars were planted in a randomized complete block design (RCBD) with 3 blocks in each sowing date. Four seeds were planted at the spacing of 60 cm between rows and 15 cm between holes. At V1~V2 stage, thinning plants and remain only two plants in a hole. A plot area was 9.6 m2 (4m long and four rows). Black vinyl was mulched for weeds and soil moisture control. Compost (10 ton/ha) and fertilizers (N-P-K, 30-30-34 kg/ha) were applied before plowing and follow the cultivation methods of Agricultural Science Technology Standards for Investigation (RDA, Jeonju, Korea).
Monitoring of soybean growth, development, and yield components
The traits like days from sowing to flowering in 50% plants (STF), days from flowering to maturity of 50% plants (FTM) were evaluated considering a state of plants population in a plot. Plant height (PH, from soil surface to the tip of the main stem), number of nodes (NN, on the main stem), number of branches (NB, on the main stem), and number of pods (NP) were measured from 10 plants selected randomly except extreme plants in a plot. The seed yield was measured from whole harvested seeds from middle area of 4.8 m2 (2 rows out of 4 rows) except outer extra 2 rows in total 9.6 m2. After that the weight was converted to kilogram per 10 are (kg/10a). 100-seed weight (HSW) was measured from 100 seeds collected randomly from each plot with three replications.
Measurement of isoflavone
Soybean seed powder (1 g) was extracted with 50% methanol (20 mL) by continuous stirring at room temperature for 24 h, followed by centrifugation (13,500 rpm, 10 min). The supernatant was passed through a 0.2 μm membrane filter. The isoflavone content was measured using a high-performance liquid chromatography (HPLC) system (Ultimate 3000 HPLC, Dionex, Sunnyvale, CA, USA) following the method described (Dhungana et al., 2021; Lee et al., 2013). The HPLC conditions were as follows—column: LiChrospher ® RP-C18 (5 μm, 4 mm × 125 mm) (BGB Analytik AG; Boeckten, Switzerland), solvent A: distilled water with 0.1% acetic acid, solvent B: acetonitrile with 0.1% acetic acid, flow rate: 1 mL/min, detector: UV-vis detector, sample injection amount: 10 μL.
Seed visual quality determination
The number of diseased seeds were counted in randomly selected from 100 seeds in three replicates. The seed visual quality was determined in the bulk seeds harvested in 2014. Three diseases such as purple seed stain (Cercospora kikuchii), Phomopsis seed decay (Diaporthe sp.), and seed discoloration caused by Soybean Mosaic Virus (Kasai et al., 2009; Pacumbaba, 1995) were identified by the seed appearance.
Statistical analysis
The data were analyzed with analysis of variance using R Program (R Core Team, 2021). The analysis of variance (ANOVA) was analyzed for 12 traits by sources of year (Y), cultivars (C), sowing date (S), Y×C, C×S, and Y×C×S. The significant differences among treatment means were determined at p < 0.05 level using the Duncan Multiple Range Test. Ten plants from each block were considered for measuring traits like days from sowing to flowering, days from flowering to maturity, plant height, number of nodes, number of branches, and number of pods for statistical analysis. The other measurements like seed yield, seed isoflavone content, seed visual quality, and 100-seed weight were conducted in three replications.
RESULTS
Effects of sowing date on flowering (STF) and maturing (FTM) duration
A gradual reduction on STF was found in all three cultivars from May 5 through June 25, however, the pattern was not consistent for the last three sowing dates (Fig. 1A). Although the STF for first sowing of Daewonkong (57 d), Pungsannamulkong (71 d), and Hwangkeumol (47 d) relatively different, it was almost same i.e., 33, 36, and 31, respectively, for the last sowing. Year, cultivar, sowing date, and their interactions were significant (p<0.0001) factors affecting STF (Supplementary Table S1).
Unlike Daewonkong, the other two cultivars Punsannamulkong and Hwangkeumol did not show a gradual reduction on FTM from May 5 through July 5 (Fig. 1B). Pungsannamulkong had the highest STF but had lower FTM than that of Daewonkong. The average time from flowering to maturity was 81.4, 66.5, and 60.1 d for Daewonkong, Pungsannamulkong, and Hwangkeumol, respectively, with the ranges of 67−106.5, 60−73.5, and 53.8−67 d. The FTM of Pungsannamulkong for the first five sowing dates was not significantly different. Surprisingly, the FTM of Hwangkeumol for the third sowing (67 d) was significantly higher than the first (63 d) and second (61 d) sowing, and FTM of the second (61 d) and last (60 d) sowing was not significantly different. Like STF, year, cultivar, sowing date, and their interactions were significant (p<0.0002) factors affecting FTM (Supplementary Table S1).

Fig. 1.
Variation in days from sowing to flowering (A) and flowering to maturity (B) in three cultivars Daewonkong, Pungsannamulkong, and Hwangkeumol due to differences in sowing dates. The numbers followed by colored blocks denote months and days of seed sowing dates i.e., May 5, May 15, May 25, June 5, June 15, June 25, July 5, July 15, and July 25 in 2013 and 2014. Different letters above the bars of the same cultivar indicate significant differences.
Effects of sowing date on plant height (PH)
The variation in PH of three cultivars showed similar pattern for the first to third planting dates. The PH differences between the early- and late-sown soybeans of late (Daewonkong and Punsannamulkong) maturing cultivars was higher than those of early maturing (Hwangkeumol) (Fig. 2). The PH of early (38 cm) and late (39 cm) maturing cultivars sown on July 25 was almost equal.

Fig. 2.
Variation in plant height in three cultivars Daewonkong, Pungsannamulkong, and Hwangkeumol due to differences in sowing dates. The numbers followed by colored blocks denote months and days of seed sowing dates i.e., May 5, May 15, May 25, June 5, June 15, June 25, July 5, July 15, and July 25 in 2013 and 2014. Different letters above the bars of the same cultivar indicate significant differences.
Effects of sowing date on the number of nodes, branches, and pods
The pattern of NN variation was roughly similar in Daewonkong and Punsannamulkong with higher number in the early-sown and lower in the late-sown treatments, however, it was inconsistent for the early maturing cultivar Hwangkeumol (Fig. 3A). The highest NN in Hwangkeumol was found with third planting date (13) i.e., May 25 and soybeans sown after this date had significantly equal NN (9−11).
The variation in NB with the sowing date did not show consistent patterns in either cultivar i.e., the patterns were random — early-sown soybeans also had fewer branches as well as late-sown soybeans had more branches and vice-versa. Surprisingly, soybeans sown on sixth date (June 25) had higher NB compared to some of the early-sown treatments (Fig. 3B). Interestingly, the plants with higher NB obtained on this date of sowing did not find to produce relatively more pods (Fig. 3C), indicating not all branches were good pod-bearer.
The late maturing cultivar Pungsannamulkong produced substantially higher NP, especially the early-sown soybeans, than the other two cultivars did with the same sowing dates. The variation in NP among the nine sowing dates was much higher in Pungsannamulkong than Daewonkong and Hwangkeumol (Fig. 3C).

Fig. 3.
Variation in number of nodes (A), branches (B), and pods (C) in three cultivars Daewonkong, Pungsannamulkong, and Hwangkeumol due to differences in sowing dates. The numbers followed by colored blocks denote months and days of seed sowing dates i.e., May 5, May 15, May 25, June 5, June 15, June 25, July 5, July 15, and July 25 in 2013 and 2014. Different letters above the bars of the same cultivar indicate significant differences.
Effects of sowing date on seed yield and 100-seed weight (HSW)
The pattern of seed yield variations among three cultivars differed on nine sowing dates (Fig. 4A). Daewonkong showed the significantly lowest seed yield with July 25 (1875 kg/ha), followed by May 5 (2099 kg/ha) and July 15 (2431 kg/ha) planting. The other six planting dates produced significantly equal seed yield. In the case of late maturing cultivar Pungsannamulkong, the first four planting (May 5, May 15, May 25, and June 5) had the significantly highest (3106−3901 kg/ha) seed yield, followed by June 15, June 25 and July 5 planting with medium (2621−2732 kg/ha) seed yield, and the last two sowing (July 15 and 25) with the lowest (1605−1765 kg/ha) seed yield. The highest yield reduction due to sowing date variation was found in Pungsannamulkong compared to the other two cultivars. The early maturing cultivar Hwangkeumol showed the lowest (1453 kg/ha) seed yield with the last planting (July 25), whereas the other planting dates had significantly indifferent (2197−2599 kg/ha) seed yields.
Although the overall pattern of HSW variations among the nine sowing dates was roughly similar in Daewonkong and Hwangkeumol with the heavier seeds in early-sown treatments, it was nearly reverse in Pungsannamulkong with the heaviest seeds in eighth, followed by the ninth sowing dates (Fig. 4B). The late maturing cultivar Pungsannamulkong produced substantially higher NP, especially the early-sown soybeans, than the other two cultivars did with the same sowing dates. The variation in NP among the nine sowing dates was much higher in Pungsannamulkong than Daewonkong and Hwangkeumol (Fig. 3C).

Fig. 4.
Variation in seed yield (A) and 100-seed weight (B) in three cultivars Daewonkong, Pungsannamulkong, and Hwangkeumol due to differences in sowing dates. The numbers followed by colored blocks denote months and days of seed sowing dates i.e., May 5, May 15, May 25, June 5, June 15, June 25, July 5, July 15, and July 25 in 2013 and 2014. Different letters above the bars of the same cultivar indicate significant differences.
Isoflavone content variation by sowing date
A gradual rise in the total isoflavone content after third sowing date (May 25) was observed in Daewonkong, however, two decline in Pungsannamulkong and one decline in Hwangkeumol were found before the last two sowing dates (Fig. 5). In the case of Daewonkong, the lowest (1035 μg/g) and highest (2173 μg/g) total isoflavone content were measured in the soybeans planted on May 5 and July 25, whereas in the case of Pungsannamulkong and Hwangkeumol were May 25 and July 25, respectively. Regarding the variation in the individual isoflavone components, the content of glycitin was less affected (146−225 μg/g in Daewonkong, 104−160 μg/g in Pungsannamulkong, and 65−110 μg/g in Hwangkeumol) as compared to daidzin (303−786 μg/g in Daewonkong, 283−775 μg/g in Pungsannamulkong, and 157−546 μg/g in Hwangkeumol) and genistin (586−1210 μg/g in Daewonkong, 437−884 μg/g in Pungsannamulkong, and 202−713 μg/g in Hwangkeumol) by sowing date.
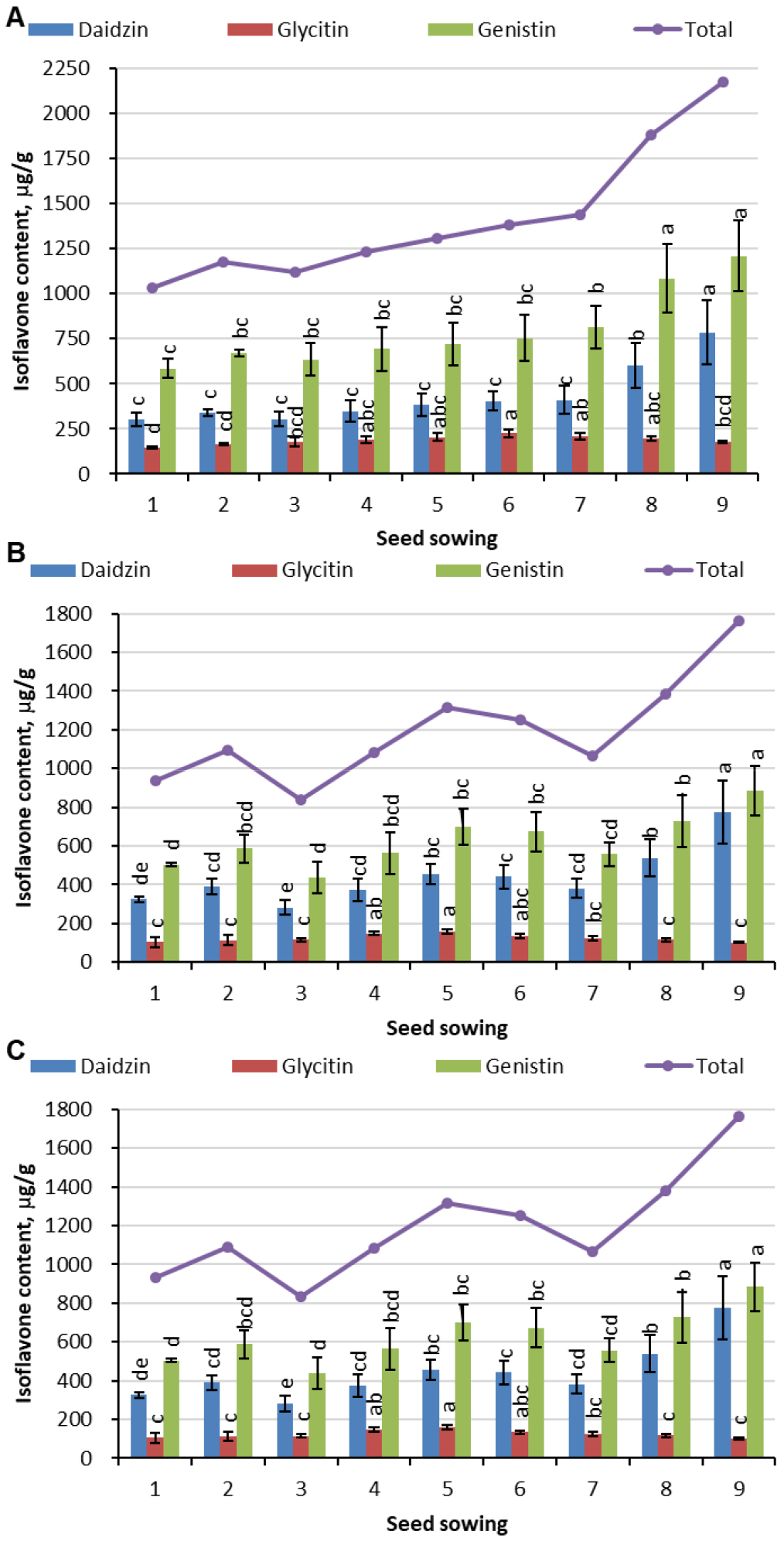
Fig. 5.
Isoflavone content in Daewonkong (A), Pungsannamulkong (B), Hwangkeumol (C) sown on nine different dates i.e., May 5, May 15, May 25, June 5, June 15, June 25, July 5, July 15, and July 25 in 2013 and 2014 indicated by 1 through 9, respectively. Different letters above the bars of the same isoflavone components across nine different sowing dates indicate significant differences.
Seed visual quality/disease infection
The effect of planting date on the seed visual quality, defined as diseased seeds, is depicted in Fig. 6. Occurrence of seed diseases due to variation in sowing date differed with cultivar. In the case of Daewonkong, Cercospora purple seed stain (CPSS) was observed in the early-sown soybeans i.e., May 5 through June 15, the highest CPSS was found in Hwangkeumol with June 15 planting (10%), whereas Pungsannamulkong produced roughly equal CPSS-affected seeds in all cases (with the highest value of 3.67% for May 15 planting). Phomopsis seed decay (PSD) was relatively high in the early-planted Pungsannamulkong but was comparatively high in the late-planted Hwangkeumol. Daewonkong produced relatively equal proportions of PSD-affected seeds for five different sowing dates with the highest value obtained for May 5 (8.17%), followed by July 5 (6.17%) plantings. The occurrence of seed discoloration due to Soybean Mosaic Virus (SMV) was very low in all three cultivars compared to the other two diseases. SMV-affected seed discoloration was found in Daewonkong only with the last two plantings (July 15 and July 25) and that in Punsannamulkong with the first (May 5) planting. In the case of Hwangkeumol, SMV seed discoloration was found in both early- and late-sown soybeans, but not in them sown from June 15 through July 5.

Fig. 6.
Percentage of seeds infected with Cercospora blight, brown spot, and pod and stem blight in Daewonkong (A), Pungsannamulkong (B), Hwangkeumol (C) sown on nine different dates i.e., June 5 (5.5) through July 25 (7.25) in 2013 and 2014. Different letters above the bars of the same disease groups indicate significant differences.
Correlation between sowing date and agronomic traits and seed quality
Eight agronomic traits, STF (r=-0.97, p<0.001), FTM (r=-0.95, p<0.001), PH (r=-0.87, p<0.001), NN (r=-0.91, p<0.001), NB (r=-0.85, p<0.001), NP (r=-0.91, p<0.001), HSW (r=-0.71, p<0.001), and yield (-r=0.79, p<0.05) had significant negative correlation with sowing date, but total isoflavone (r=0.86, p<0.01) and component of it, as daidzin (r=0.84, p<0.01) and genistin (r=0.86, p<0.01) showed significant positive correlation with sowing date. On the other hand, correlation of Glycitin, CPSS, PSD, SD, and TSD with sowing date were not significant. (Table 1). The results mean that delayed sowing date affect growth smaller and isoflavone content higher.
Table 1.
Pearson correlation matrix among 11 agronomic traits, isoflavone components, and seed diseases.
DISCUSSION
Soybean flowering is regulated by temperature and photoperiod, with cultivars varying from qualitative to quantitative short-day plants (Hadley et al., 1984). Flowering and subsequent reproductive development in soybean is influenced by the interaction of photoperiod and temperature (Agele et al., 2004; Cober et al., 2001). In the cases of shorter day lengths during sowing dates than a threshold value for a particular cultivar, its flowering will appear to be indifferent to day length (Constable & Rose 1988). Increment in days to flowering was found to be related to increased photoperiod (when photoperiod is longer than the critical photoperiod) and decreased temperature (Hadley et al., 1984; Major et al., 1975). Our results were in agreement with the previous finding i.e., the early-sown soybeans experienced increased photoperiod and decreased temperature, resulting in increase in days to flowering compared to the later-sown ones. As the previous report, shorter days triggered the late-maturing genotype, which is more sensitive to day length, Pungsannamulkong to flower more rapidly in the later-sown soybeans (Lawn & Byth, 1973).
Soybean plant height increased with planting date when sown early, however started decreasing with plantings after early June. A similar pattern was found in previous research that soybeans sown before June reached a greater plant height when compared to those planted after June (Beatty et al., 1982; Sweeney et al., 1995). The photoperiod and temperature for early and late sowing vary greatly and final plant heights may be affected by these factors (Alliprandini et al., 2009; Zhang et al., 2017). Schaik & Probst (1958) found that increasing temperature and longer photoperiod increased the plant height of two soybean cultivars Clark and Midwest. Clark and Daewonkong come under same maturity group. The effect of photoperiod and temperature was less determinant in the early maturing Hwangkeumol.
Later maturing cultivars have longer vegetative growth periods and more nodes than earlier cultivars (Wilcox et al., 1995; Egli & Bruening, 2000), however, later sowing with shorter vegetative growth periods often produce smaller number of nodes than earlier sowing (Bastidas et al., 2008; Egli et al., 1985).
Poor branch development in late-planted soybeans is one of the causes of yield reductions (Settimi & Board, 1988). They observed that the photoperiod of a later sown soybeans restricts branch development at the lower nodes, which produced higher yields than the branches produced on upper nodes, by promotion of early flowering. The photoperiod study revealed that shorter reproductive period (R1 to R5 stages) and the associated decline in branch number were photoperiodically induced phenomena (Board & Settimi, 1986), both pre- and post-flowering day length regimes at a late planting date were involved in these events.
Normally, the number of nodes is associated with yield, however, the environment may affect the number of nodes on pod production and survival (Egli, 2013). Yield is the key factor to a soybean producer and was significantly affected by planting date. Reduced yield due to late sowing was explained primarily by reduction in numbers of nodes, which were in agreement with previous reports (Bastidas et al., 2008; Kumagai & Takahashi, 2020). As planting delays, the growth and developmental time of soybean crops decreases, potentially producing small plants with reduced yield (Bastidas et al., 2008; Thomas & Raper 1976; Wilcox & Sediyama, 1981). The higher yields in the early-planting of late maturing soybeans was in agreement with Malik et al. (2007). The higher yield for the earlier plantings was most likely due to optimal growing conditions and sufficient time for plant growth and development. The yield of early maturing cultivar Hwangkeumol was rather unaffected due to planting date than the late maturing cultivar Pungsannamulkong. The effect of photoperiod and temperature on yield was less determinant in Hwangkeumol.
Although the HSW of Daewonkong and Hwangkeumol was generally lighter in the later planting, Pungsannamulkong produced heavier seeds on the last two plantings. In a previous study (Jung et al., 2012), the HSW of Daewonkong planted on May 25 was heavier than that planted on June 25. Several previous studies have reported similar results of lighter seeds with delayed sowing (Beatty et al., 1982; Elmore, 1990). Similarly, and in another previous study, we observed a reduction in seed weight with planting date according to cultivar (Kandil et al., 2013; Kang et al., 1998). The yield reduction in later sowing was not associated with HSW (De Bruin & Pedersen, 2008; Kumagai & Takahashi, 2020), however, one of the reasons for the increased seed yield was attributed to the increase in HSW (Kandil et al., 2013). The previous results were in agreement with that found in the present study.
The correlations among different yield components found in the present study were in agreement with several previous reports. The seed yield had significant positive correlation with the PH (Borowska & Prusiński, 2021; Ferrari et al., 2018; Mandić et al., 2020), NN and NP (Arshad et al., 2006; Mandić et al., 2020). It also found positive effect of PN on the yield of soybean (Iqbal et al., 2004). A non-significant positive correlation between PH and HSW and significant positive correlation between PH and PH were observed in soybean (Sarutayophat, 2012). In the same study (Sarutayophat, 2012), a non-significant negative correlation was found between HSW and PN which was significant in our study.
The higher isoflavone concentrations in the soybeans sown in July than in May was hypothesized to be resulted due to lower temperatures and higher precipitation during pod development and seed-filling stages (Kim et al., 2012). Other researchers also agree to the reasons of low temperatures and high precipitation for the higher isoflavone content on the delayed planting soybeans (Caldwell et al., 2005; Lozovaya et al., 2005; Tsukamoto et al., 1995). Moreover, the extent of variations in seed isoflavone concentrations in response to changes in temperature and soil moisture was greatly cultivar dependent (Lozovaya et al., 2005). Our results corroborate with that of previous reports. While investigating the isoflavone content in six soybean cultivars grown at eight locations, genotype, genotype×year, genotype× location, and genotype×year×location interactions all influenced the isoflavone concentration (Hoeck et al., 2000).
The variation in CPSS is suggested to be influenced by the pod age at the time of infection rather than the difference in the length of flowering and maturing of soybeans (Kilpatrick, 1957; Murakishi, 1951; Roy, 1976). Reports showed that the early planting could result in plants developing and maturing under conditions more favorable for CPSS and other fungal pathogens (Li et al., 2019; Ploper et al., 1992; Wilcox et al., 1985). No significant interaction between maturity group and CPSS although there were significant differences in the percent CPSS among genotypes within a maturity group, suggesting the occurrence of CPSS was independent of the genotypes’ maturity (Li et al., 2019). The random variations in CPSS observed among three cultivars with different planting dates found in the present study were supported by the previous reports. The PSD is reported to be more prevalent under high soil moisture/rainfall during seed maturity (Thomison et al., 1987). The early sowing of late (Daewonkong and Punsannamulkong) maturing cultivars and late sowing of early maturing cultivar Hwangkeumol showed relatively high PSD infection corresponding to the high soil moisture/rainfall during harvest (Supplementary Fig. S1 and S2). Similar results of varied degree of PSD affected by planted date and cultivar were also found (Wrather, 1996). Although there are no references to support the effect of planting dates on the seed discoloration due to SMV, our result implied that SMV seed discoloration is not associated with plating date and maturity duration of soybeans.
Unexpected rainfall, occurring over extended periods, coupled with a double-cropping system involving winter crops such as wheat, is an important factor contributing to the stable production of soybeans. June 20 as the optimum sowing date for highest yield, and it remained economically viable until July 20. However, considering the diverse possibilities of damage from the rainy season in the current unpredictable climate, early sowing is considered one of the solutions (Lee et al., 2019). While early sowing can enhance growth volume and the potential for high yield, it also increases the risk of lodging and disease. In the case of double cropping with winter crops, the sowing date can be delayed until mid-July to accommodate extended rainy season. However, it is important to note that in late sowing, there is a decrease in main agronomic traits, necessitating a higher planting density (Park et al., 2014, 2015). Considering the growth response of soybeans in this study, suitable cultivation methods can be developed for both early and late sowing dates.
CONCLUSIONS
The impact of adverse weather conditions on field crop cultivation, with a particular focus on regulating sowing dates for soybeans. Soybeans, being both photophilic and thermophilic, are affected by short-day photoperiodism. We conducted an experiment to determine the optimal sowing time to mitigate the effects of severe weather on soybean yield. The research, conducted with three Korean soybean cultivars of varying maturity, explores growth, development, yield components, isoflavone levels, and seed visual quality. The three cultivars exhibited the highest figures in the flowering, maturing duration, and number of node when sown earlier, but variations in 100-seed weight and seed yield were observed. Also, delaying the sowing dates for all three cultivars resulted in increased isoflavone levels. While the seed yield was approximately 3,800 kg/ha for earlier planting, the largest 100-seed weight (13 g) was recorded on July 15 for Pungsannamulkong. Hwangkeumol demonstrated the highest seed yield on July 5 and the 100-seed weight was consistently high until Jun 15. Consequently, we recommend determining the sowing time for cultivars based on regional and environmental considerations. The study highlights that later sowing dates contribute to higher isoflavone levels, accompanied by significant variation in other components based on the sowing date. The research provides valuable information and insights into the agronomical response of representative cultivars with early and late growth characteristics in Korea based on different sowing dates.




