Introduction
Materials and Methods
Sample Preparation
Sample Extraction
Determination of the DPPH Radical Scavenging Activity
Determination of the Total Phenol Content
Determination of the Reducing Power Activity
Determination of the ABTS Radical Scavenging Activity
Statistical Analysis
Results and Discussion
Seed Coat Color
Conclusion
Introduction
The natural antioxidants such as ascorbic acid, tocopherols, carotenoids and phenolic compounds (polyphenols) are present in fruits, legumes, vegetables and whole grains (Choi et al., 2007). Among the whole grains, Iqbal et al. (2005) identified that there were several compounds with antioxidant activity in rice, including phenolic compounds, tocopherols, tocotrienols and γ-oryzanol. Whole grain rice is recognized for its nutritional superiority because it contains embryo and bran, which have various nutritional and bioactive compounds (Jones et al., 2002). However, the concentration of these compounds in the grain were reduced during the milling process. Therefore, in these days, the use of brown rice (BR) has increased in standard diets and those diets were catered to people with celiac disease or allergies to other cereals. In addition, germination can improve the nutritional quality of cereals (Ochanda et al., 2010). The germinated brown rice (GBR) grains provides higher nutritional and functional values since they are associated with the quality and quantity of their nutrients, biologically active compounds and antioxidant potential. Thus, there is an increasing trend focusing on the use of GBR in the formulation of high quality health products. Moreover, in pigmented rice, there are naturally occurring color substances that belong to the flavonoid group called anthocyanins. Positive health effects of the pigments present in the bran layer of rice have been reported. A commonly found anothocyanin in color possess a free radical scavenging activity (Oki et al., 2002).
The term weedy rice generally includes all the species of genus Oryza which behave as weeds (Parker & Dean, 1976; Ferrero & Finassi, 1995). Many weedy rice plants share most of the features of the two cultivated species Oryza sativa and O. glaberrima (Khush, 1997) and can also adapt to a wide range of environmental conditions and show a wide variability of anatomical, biological and physiological features (Chung & Paek, 2003; Craigmiles, 1978; Kwon et al., 1992; Tang et al., 1997, Vaughan et al., 2001). Weedy rice (Oryza spp.) also known as red rice because the red pigmentation is a dominant character. In Korea, weedy rice (Oryza sativa L.; locally called ‘Aengmi’ and ‘Shareibyeo’) survive in farmers’ fields as a weedy form and is distributed in all parts of the country (Hara, 1942; Suh et al., 1992). Weedy rice from South Korea had long and brown awn, red pericarp, and very short grain and flag leaf characters (Zhang et al., 2017). Among the Korean weedy rice, ‘Sharei-rice’ is originated from Ganghwa Island, thus geographically isolated from other weedy populations (Chung & Park, 2010). Sharei-rice is considered a useful germplasm that might have unique characteristics because it successfully acclimatized and adapted to the isolated natural growing conditions (Heu et al., 1990; Suh et al., 1992). Therefore, the hypotheses of this study were that Sharei-rice might have a unique functional substance for high antioxidant activity, which might be enhanced under germination process. Based on the hypotheses, the aims of this study were to examine the antioxidant activities of Sharei-rice germplasm compared to a check cultivar, to screen the germplasm for high antioxidant activities in germination and non-germination conditions, and finally to select an accession of Sharei-rice showing the highest antioxidant activity, which will be used as a potentially functional substance material in further research.
Materials and Methods
Sample Preparation
One hundred and ninety nine accessions of Sharei-rice germplasm were used. “Sharei” is a proper name calling for the weedy rice that is native to Ganghwa Island in South Korea. They were received from the National Agrobiodiversity Center of Rural Development Administration (RDA) of Korea, and were regenerated in the experimental field in Chonbuk National University. The seed coat color was directly identified with the visual observation after removing the husk to obtain the whole grain. For the first assay of accessions to check the maximum antioxidant activity, DPPH radical scavenging activity was measured. The 19 accessions screened based on the DPPH radical scavenging activity were then screened by checking their total phenol content, DPPH radical scavenging activity, reducing power activity, and ABTS radical scavenging activity. A Korean bred rice cultivar, Sindongjin (SDJ), was used as a check variety in this study.
Sample Extraction
Brown rice seeds (50 g) were cleaned. The half of seeds (25 g) were sterilized with 0.1% sodium hypochlorite solution (1:5 w/v) at 30°C for 30 min. Then, the seeds were washed thoroughly in the deionized water and soaked in water (1:5 w/v) for 24 hours at 35°C. The drained rice seeds were placed evenly in a plastic tray overlaid with the moist blotting papers and then the second tray was placed below the first tray. In order to keep the blotting paper wet by capillarity, the upper tray had holes to absorb the water and the lower tray contained the water. The seeds were incubated at 30°C for 48 hours for the germ to develop. After the incubation period, germinated rice seeds were not dried in the oven in order to prevent losing and destroying of the chemical compounds. They were dry freezed in liquid nitrogen solution and then were kept for 4 days in dry freezer machine. And then the dry freezed and non-germinated brown rice seeds (25 g) were grounded by the Speed Control Auto mill (Toklen, Inc). These rice flours were used to detect the antioxidant activity. The rice flours of each sample were extracted with 70% methanol (1:10 w/v) for 24 hours in the shaking incubator at 50°C and 150 rpm. The resulting methanol extracts were filtered. Then, the methanol layer in each tube was separated by centrifugation at 13,000 rpm for 20 min at 4°C. The supernatant was used for the determination of antioxidant activity for DPPH assay. For total phenol determination, reducing power activity and ABTS radical scavenging activity assays, the filtrates were evaporated with Eyela Rotary evaporator and end up to 50 ml and then freeze-dried. The freeze-dried extracted powders were stored at -20°C until used for further analysis.
Determination of the DPPH Radical Scavenging Activity
The free radical-scavenging activity of rice extract was evaluated using the stable radical DPPH, according to the method of Blosis (1958). Briefly, the DPPH reagent (0.0059 g) was dissolved in 100 ml of methanol for preparing the DPPH reagent solution. Then, extract solution (300 μl) of each sample was transferred into the test tube and mixed with 1.7 ml of 0.15 mM DPPH in methanol. The mixture solution was shaken vigorously by using vortex mixture and then was allowed to stand for 30 min at the room temperature. The control sample was prepared without adding the extract solution. The absorbance of the sample was monitored at 517 nm using a Genesys R-20 spectrophotometer, using methanol for base line corrections (blank). The inhibition percentage of the absorbance of the DPPH solution was calculated using the following equation.
DPPH radical scavenging activity (%) = [1- (As/Ac)] x 100 (1)
Where Ac = the absorbance of DPPH of the control, As the absorbance of DPPH of the extracted sample. The experiment was carried out in triplicated.
Determination of the Total Phenol Content
The total phenolic content was determined using the Folin- Ciocalteu reagent, according to method of Swine and Hillis (1959). The 0.2 ml of the extract solution (0.01 g per 10 ml of the deionized water) was transferred into a test tube containing 0.4 ml of 10% 2N Folin-ciocalteu and then mixed thoroughly and incubated for 6 min at room temperature. And then the mixture solution was added by 0.8 ml of 10% sodium carbonate. The mixture solution was mixed using vortex mixer and then allowed to stand for 60 min in the dark. The absorbance of rice extracts were measured in spectrophotometer at 750 nm. The blank was consisted of a solution only with the Folin-Ciocalteu reagent (without the extract). Total phenolic content was calculated from a calibration curve obtained with the standard gallic acid. This experience was carried out in triplicate. For gallic acid standard curve, 0.02 g gallic acid was weighted and mixed with 100 mL deionized water using 100 mL volumetric flask. The gallic acid concentrations were 0, 20, 40, 60, 80, 100 ppm. Then, 0.2 ml of each standard concentration was added to corresponding tubes and mixed with 0.4 ml of 2N Folin-Ciocalteu reagent. The mixed solution was vortexed and incubated for 6 min at room temperature. After incubation, 0.8 ml of 10% sodium carbonate was added more and vortexed again. The mixed solution was kept at room temperature for 60 min in the dark condition and was read the absorbance at 750 nm vs the blank. The results of the total phenolic compounds were expressed as mg gallic acid equivalents (GAE) per 100 g of rice flour. The calibration curve of gallic acid as the standard to generate the straight line equation was y = 0.4022x – 0.3185, R2 = 0.9991.
Determination of the Reducing Power Activity
The reducing power was determined according to the method of Oyaizu (1986). The methanolic extract (2.5 ml) was mixed with 2.5 ml of 0.2 M sodium phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min in water bath. After that, 2.5 ml of 10% trichloroacetic acid (w/v) was added, and then the mixture was centrifuged at 3,000 rpm for 10 min. The upper layer (5 ml) was mixed with 5 ml of the deionized water and 1 ml of 0.1% ferric chloride, and the absorbance was measured at 700 nm: the higher absorbance indicates the higher reducing power. The assay was carried out in triplicate.
Determination of the ABTS Radical Scavenging Activity
The spectrophotometric analysis of ABTS+ (2, 2’-azinobis-3- ethylbenzothiazoline- 6-sulfonic acid) scavenging activity was determined according to the method of Re et al., 1999. The ABTS radical cation (ABTS•+) was produced by reaction of 7 mM stock solution of ABTS with 2.45 mM potassium persulfate and allowing the mixture to stand in dark at room temperature for 12 hr before use. The ABTS•+ solution was diluted with ethanol to give an absorbance of 0.7 ± 0.02 at 734 nm. The extracts (0.1 ml) was allowed to react with 0.9 ml of the ABTS•+ solution and the absorbance was measured at 734 nm after 6 min and the scavenging capacity of test compounds was calculated using the following equation.
ABTS + scavenging activity (%) = [1- (As/Ac)] x 100 (2)
Where Ac = the absorbance of the control (blank), As the absorbance of the remaining ABTS + in the presence of scavenger. The experiment was carried out in triplicated.
Statistical Analysis
All the parameters were measured in triplicate, and presented as means ± standard deviation (SD). Data analysis was performed with SAS program version 9.1 (SAS Institute Inc., Cary, NC, USA). Significant differences among the treatments were found using Analysis of Variance (ANOVA), followed by Duncan’s Multiple Range Test. The Student’s t test was analyzed to test the difference between the germination and non-germination treatments. Pearson correlation among the results from four assays (DPPH, ABTS, reducing power activity and total phenol determination) was also analyzed. Statistical significance was defined at level of P < 0.05.
Results and Discussion
Seed Coat Color
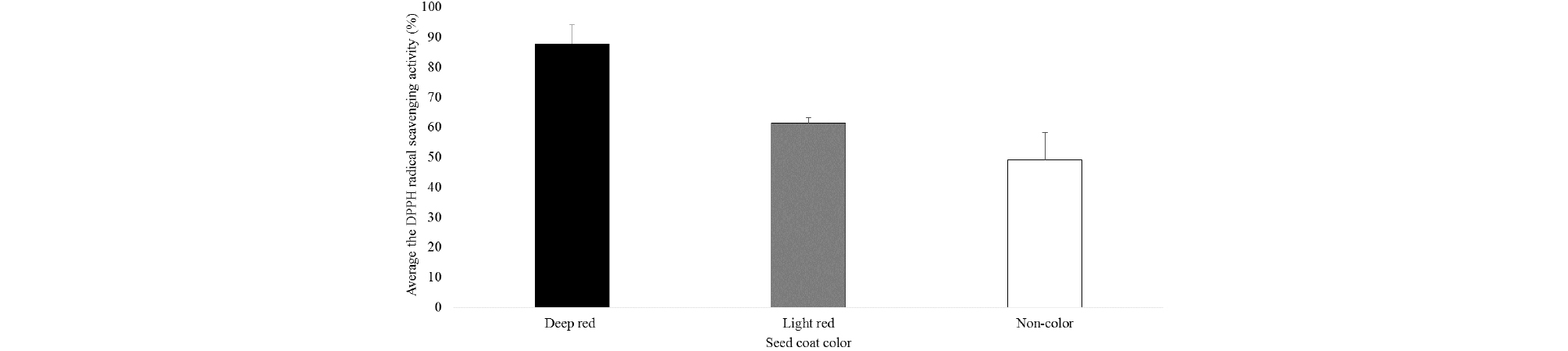
The seed coat color of 199 accessions of Sharei-rice were varied. The seed coat colors of the germplasm consist of 3 kinds of colors; deep red, light red and non-color, which were 171 accessions (86%), 2 accessions (1%) and 26 accessions (13%), respectively (Fig. 1). Heu et al. (1990) and Suh et al. (1992) reported that the seed coat color of Korean weedy rice varied from dark purple, red and brown to white or colorless. According to this result, red seed coat color was dominate character in Korea-native weedy brown rice. Wirjahardja et al. (1983) also reported that weedy rice grains frequently had a red pigmented testa and the red pigmentation was a dominant character in weedy rice. This red colored seed coat may be formed due to the combination of Rc and Rd genes that were responsible for the accumulation of pigment in pericarp of rice (Nagao et al., 1957; Sweeney et al., 2006; Furukawa et al., 2007). Prathepha (2009) and Ziska et al. (2015) also mentioned that weedy rice biotypes showed variability in the pericarp color, and biotypes without red color were also found. The frequency distributions of the of DPPH radical scavenging activity of Sharei-rice germplasm were shown in the Fig. 1. Among the germplasm, 19 germplasm showed very high DPPH radical scavenging activity that is higher than the 91%.
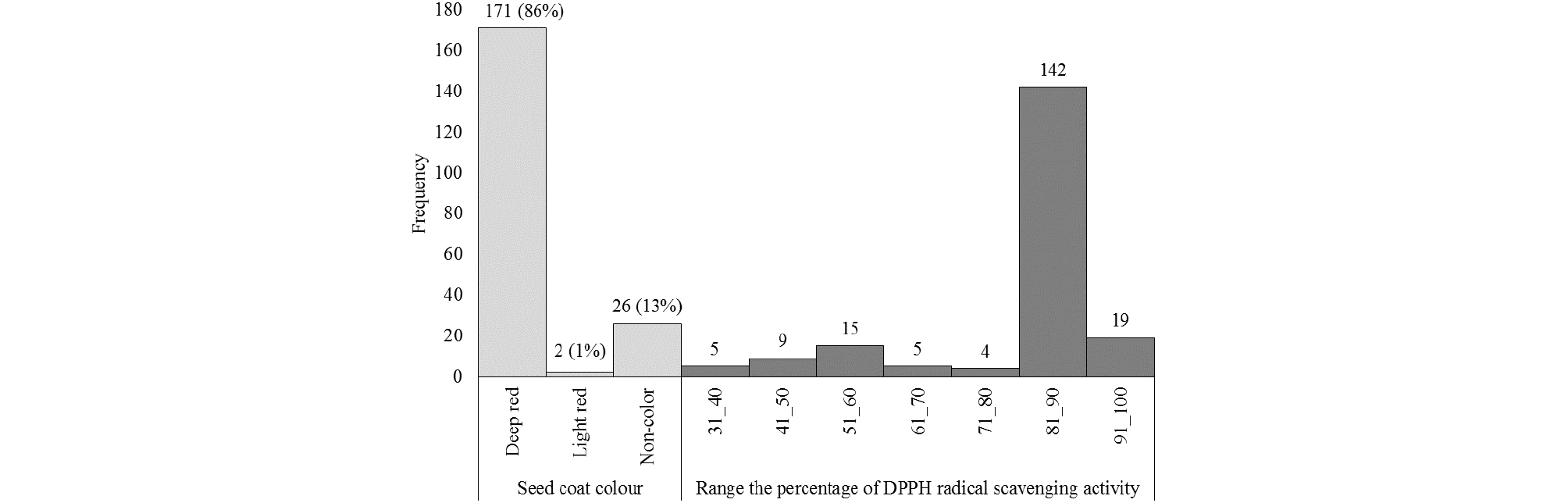
Fig. 1.
Frequency distribution of seed coat color and the DPPH radical scavenging activity of brown rice in 199 accessions of Sharei-rice germplasm. Measurements of the DPPH radical scavenging activity were rounded up to integers to determine the frequency. Note that all the numbers in the parentheses are the percentages of seed coat color of total accessions.
The DPPH radical scavenging activity values of all the selected lines varied in the range from 31.0 to 91.7% and mean value was 82.5 ± 14.8%. Depend on the seed coat color, the higher average DPPH radical scavenging activity (87.8 ± 6.3%) was found in the deep red colored rice followed by the light red (61.4 ± 1.8%) and the non-colored seed coat (49.1 ± 9.0%), respectively (Fig. 2). The result showed that the deep red colored accessions had higher antioxidant activity. Usually, grains with red and black pericarp presented higher antioxidant activity than those with light brown pericarp color (Nam et al., 2005). Nam et al. (2006) reported that, with some exceptions, extracts from the pigmented rice seeds had the higher antioxidative activity than did the non-pigmented seeds. Besides, this study showed that there were some variation of the antioxidant activities not only among the different seed coat color but also within the similar seed coat color.
Among the Sharei-rice germplasm, 19 accessions showing high DPPH radical scavenging activity were selected as shown in Table 1. The DPPH radical scavenging activities of the selected accessions were higher than or equal 91%. Moreover, all the selected germplasm had red colored seed coat.
Table 1. The DPPH radical scavenging activity in the first screened Sharei-rice germplasm.
The antioxidant activities of the first selected 19 accessions and a cultivated rice (SDJ) were shown in Table 2. Under the germination and non-germination conditions, the antioxidant activity of each accessions in the selected germplasm was significantly much higher than that of the cultivated rice (SDJ) in all assays. In other words, the measurements of BR and GBR of SDJ were the lowest that were 68.8 and 36% in DPPH, 55.1 and 61.5% in ABTS, 0.3 and 0.2 in reducing power activity and 161.8 and 166.2 mg GAE 100g-1 in total phenol contents, respectively. The antioxidant activities of selected accessions in DPPH, ABTS and Reducing Power Activity were around 1.5 to 2.5 times higher than that of SDJ. Similarly, the total phenol contents of the selected accessions were around 1.25 to 3 times higher than that of the SDJ. According to Lee et al. (2011) who studied the antioxidant capacity of 591 rice cultivars including white rice, weedy red rice and pigmented rice, blackish purple rice, pigmented rice cultivars showed twice stronger antioxidant activities than the white rice cultivars. The result showed that the higher antioxidant activities resulting the presence of a variable content of different anthocyanin were found in all red colored weedy rice accessions than that of the non-colored rice. This was also agreed with the report of the antioxidant properties of pigmented rice from Sabah, Malaysia by Lum & Chong (2012). In their report, the red rice variety had the highest DPPH radical scavenging activity. Romero et al. (2009) also mentioned that pigmented rice such as black, purple, or red rice was a good source of antioxidants because it contained anthocyanins which were effective free radical scavengers. Grains with darker pericarp color, such as red and black rice, contained higher amounts of polyphenols (Tian et al., 2004; Zhou et al., 2004). Muntana & Prasong (2010) also proved that Thai red rice cultivars possessed relatively strong antioxidant activity and the antioxidant activities in the red rice bran were largely owing to the phenolic compounds. Besides, they observed that white rice also showed the lowest both total phenolic content and antioxidant activity. Moreover, Goffman & Bergman (2004) also described that pigmented rice varieties were more phenolic acid-rich compared to non-pigmented rice varieties.
Table 2. Antioxidant activities of brown rice (BR) and germinated brown rice (GBR) in the first selected 19 accessions and a cultivated rice, Sindongjin (SDJ).
2ns non-significant difference; * Significant at the 5% level, ** Significant at the 1% level
The antioxidant activities of SDJ were not significantly difference between the germination and non-germination conditions except in DPPH assay. In DPPH assay, the antioxidant activity of SDJ was significantly decreased from 68.8 to 36.0% when the seeds were germinated.
Regarding the only selected weedy rice lines with or without germination conditions, the antioxidant activities were not much differences each other in the DPPH assay. Under germination condition, the antioxidant activities of most of the weedy rice lines were more or less slightly decreased; especially in reducing power activity and total phenol determination methods. According to the t-test, some lines were not significantly decreased but some lines were significantly decreased in antioxidant activities under germination condition. These decreases might be due to the loss of seed coat color because brown rice seeds were germinated and the color was bleached during the soaking time. Sutharut & Sudarat (2012) also found that Trolox equivalen antioxidant capacity of germinated colored rice that prepared from dehulled rice was slightly decreased when the germination time was increased. Zhanqiang et al. (2017) also mentioned that germination in red rice caused decreases of free total phenol content, antioxidant activity, and some phenolic composition.
Exceptionally, the phenol content of line no. 216 (WD3) was significantly increased from 450.5 to 510.7 mg GAE 100 g-1 when the seeds were germinated. Moreover, WD3 showed the highest antioxidant activities; 99.8% in BR and 98.9% in GBR by ABTS assay, 1.9 in BR and 1.0 in GBR by reducing power activity and 510.7 mg GAE 100 g-1 in GBR by total phenol determination.
There was variation in total phenol contents of the grains within similar testa colored rice. Goffman & Bergman (2004) also showed that there was some variation of total phenolic contents in evaluating the different genotypes. They found that total phenolic contents were ranged from 190 to 5032 mg GAE (gallic acid equivalent) 100 g-1 bran, and ranged from 25 to 535 mg GAE 100 g-1 grain, observing that for those genotypes with light brown pericarp color. Besides the difference in the content of total phenolics related to the color of the grains, variation was also observed in the content of total phenolics among the genotypes with the same pericarp color.
The correlation coefficients among DPPH, ABTS, reducing power activity, and total phenol contents in the first selected group were shown in Table 3 (non-germinated brown rice), Table 4 (germinated brown rice) and Table 5 (pool data of non-germinated and germinated brown rice). All the methods; DPPH, ABTS, and reducing power activity were positively and strongly correlated except in the DPPH assay under germination. Similarly, total phenol contents of BR and GBR were highly corrected with DPPH, ABTS and reducing power activity assays except in DPPH assay under germination. After combining the data of non-germinated and germinated brown rice, there were also found the positive and strongly correlated among all assays. Yawadio et al. (2007) mentioned that pericarp color was related to the concentration of phenolics in the grain and usually the concentration was higher in the grains with red and black pericarp. It was also reported that the concentration of total phenolics in the grain had been positively correlated with the antioxidant activity (Itani et al., 2002; Goffman & Bergman, 2004; Zhang et al., 2006; Muntana & Prasong, 2010).
Table 3. Correlation coefficients among DPPH, ABTS, reducing power activity, and total phenol contents of non-germinated brown rice in the first selected group of accessions.
| Assays | DPPH | ABTS | Reducing power activity |
| ABTS | 0.99** | ||
| Reducing power activity | 0.52* | 0.52* | |
| Total phenol | 0.59** | 0.55* | 0.85** |
Table 4. Correlation coefficients among DPPH, ABTS, reducing power activity, and total phenol contents of germinated brown rice in the first selected group (19 accessions).
| Assays | DPPH | ABTS | Reducing power activity |
| ABTS | 0.43ns | ||
| Reducing power activity | 0.35ns | 0.83** | |
| Total phenol | 0.33ns | 0.80** | 0.96** |
Table 5. Correlation coefficients among DPPH, ABTS, reducing power activity, and total phenol contents of pooled data from the germinated and non-germinated brown rice in the first selected group.
| Assays | DPPH | ABTS | Reducing Power Activity |
| ABTS | 0.80** | ||
| Reducing Power Activity | 0.55* | 0.73** | |
| Total phenol | 0.55* | 0.78** | 0.93** |
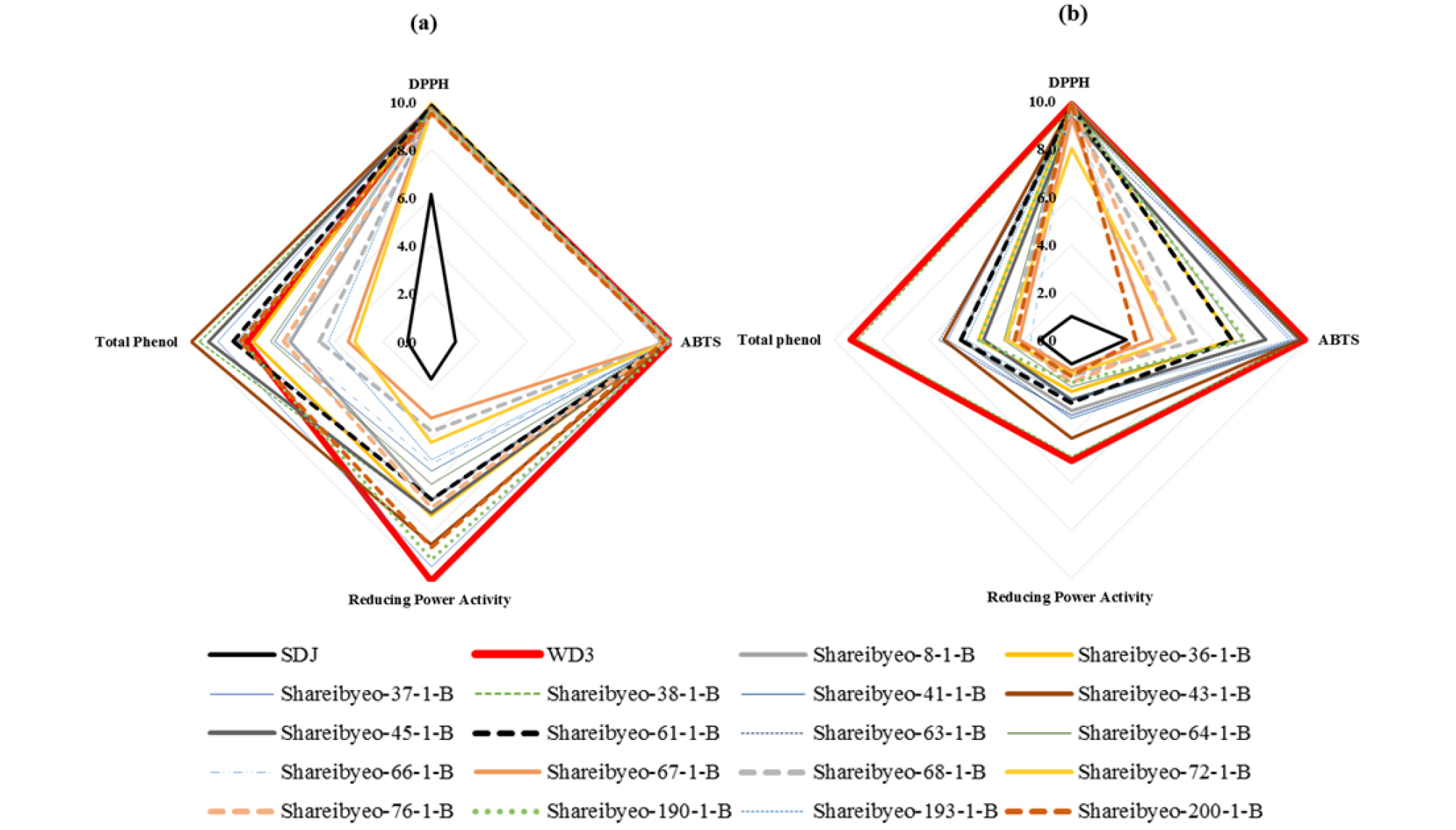
Spider plot was shown in Fig. 3 to finally select the accession having the highest antioxidant activity. The scores assigned to each factor were relatively scaled that 1 to 10 where the higher score indicated the better performance of antioxidant activity. Among the 19 accessions of Sharei-rice, WD3 had the highest score in all assays except only total phenol of BR. It showed that WD3 possessed the higher antioxidant capacity than that of other selected and cultivated ones. Oppositely, cultivated one (SDJ) had the lowest score in all assays and it showed that SDJ possessed the lower antioxidant capacity compare with Sharei-rice germplasm. According to the scores of spider plot factors, WD3 was finally selected as a potential accession to develop functional substance material for further research.
Conclusion
More than 85% of the Korea-native Sharei-rice germplasm used in the study had the red colored seed coat that had higher antioxidant activities compare to non-colored seed coat germplasm. Comparing to cultivated rice, Share rice showed much higher antioxidant activities than that of the cultivated rice. The germination of Sharei-rice did not enhance the antioxidant actitivities. Among the 199 accessions of Sharei-rice germplasm, WD3 showed that the highest antioxidant activity, therefore, it can be suggested that it could be a potential functional substance material for further research on healthy food and substance development.